Autologous Cell Therapy: Introduction
Autologous cell therapy is a technique that uses an individual's own cells or tissues to be processed and expanded outside the body and later reintroduced into the donor. This therapy provides advantages like biocompatibility, lower risk from systemic immunological reactions and disease transmission related to external grafts or cells.Autologous cell therapy finds its application in dermatology, oncology, musculoskeletal as well as autoimmune disorders. It is used in treating burns and pressure ulcers, helping wound healing, expediate postoperative healing and stabilize chronic inflammation as well.
Global Autologous Cell Therapy Market Analysis
Autologous cell therapies find their applications across several domains. It is associated with less adverse side effects that may persist due to foreign materials and incompatibility between host and outside cells. With the increasing emphasis on precision medicine, patients are showing their preference towards autologous cell therapies, which has led to significant market demand.Then autologous cell therapy market value is used effectively in wound healing. Starting in the 1980s, bioengineered skin equivalents have been effective for wound healing outcomes. However, issues like scarring, skin contracture and poor epithelialization persisted in conventional methods. Hence, researchers have been working to develop an improved and efficient method to address the side effects. In July 2023, researchers reviewed the effectiveness of an autologous cell therapy called EB-101 to treat dystrophic epidermis bullosa (RDEB) in patients. It was revealed that 81% of the wounds treated with EB-101 resulted in pain severity and healed effectively.
Around 3-5% of the world's population is affected by autoimmune diseases. Over the years, autologous cell therapies have been widely used in the treatment of autoimmune diseases. Currently, the treatment market is observing several technical innovations. In August 2023, Artiva Biotherapeutics announced that their new investigational drug got its FDA clearance to be used in the market. AlloNK® (also known as AB-101), comes in combination with rituximab for treatment of systemic lupus erythematosus (SLE) in patients affected with active lupus nephritis (LN). Autologous CAR T-cell therapies are also used in treating selective blood cancer, predominantly B cell malignancies. Owing to its wide application, autologous cell therapy market demand is anticipated to increase in the forecast period.
Global Autologous Cell Therapy Market Segmentation
Global Autologous Cell Therapy Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Autologous Cell Therapy Market
- Autologous Stem Cell Therapy
- Autologous Cellular Immunotherapies
Market Breakup by Source
- Bone Marrow
- Epidermis
- Others
Market Breakup by Application
- Oncology
- Musculoskeletal Disorder
- Blood Disorder
- Autoimmune Disease
- Others
Market Breakup by End User
- Hospitals
- Research Centres
- Cancer Treatment Centres
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Autologous Cell Therapy Market Overview
The global market for autologous cell therapy is constantly growing and witnessing significant attention as a potential treatment for many diseases. The active participation of various healthcare pioneers expediating research and development in the field. For instance, in June 2023, AstraZeneca announced an agreement with Quell Therapeutics to treat type 1 diabetes and inflammatory bowel disease with the help of developing autologous multi-modular Treg cell therapy.The United States is expected to lead the market with latest investments in the infrastructure for better research and development. Bristol Myers Squibb got its FDA clearance to set up a commercial production for the company's latest cell therapy manufacturing facility in Devens, Massachusetts. Therefore, United States will hold a huge portion of the autologous cell therapy market share in the forecast period. However, considering the technological and academic growth in the Asia Pacific, the region is anticipated to witness the fastest growth in upcoming years.
Global Autologous Cell Therapy Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Sanpower Group (Dendreon Pharmaceuticals LLC.)
- Holostem Terapie Avanzate S.r.l.
- Novartis AG
- Tego Science
- GC Biopharma (GC Cell)
- Gilead Sciences
- Johnson & Johnson (Janssen Biotech, Inc)
- Bristol Myers Squibb
- CORESTEM, Inc
- Vericel Corporation
- Opexa Therapeutics
- Lineage Cell Therapeutics, Inc.
- Pharmicell Co., Inc.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Sanpower Group (Dendreon Pharmaceuticals LLC.)
- Holostem Terapie Avanzate S.r.l.
- Novartis AG
- Tego Science
- GC Biopharma (GC Cell)
- Gilead Sciences
- Johnson & Johnson (Janssen Biotech, Inc)
- Bristol Myers Squibb
- CORESTEM, Inc
- Vericel Corporation
- Opexa Therapeutics
- Lineage Cell Therapeutics, Inc.
- Pharmicell Co., Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
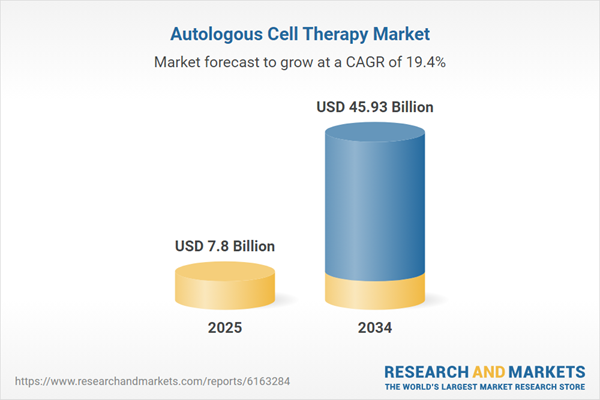
| Estimated Market Value ( USD | $ 7.8 Billion |
| Forecasted Market Value ( USD | $ 45.93 Billion |
| Compound Annual Growth Rate | 19.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |