Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
It involves tailoring treatment strategies based on the unique characteristics of an individual's cancer, including the genetic and molecular features of the tumor and the patient's immune system. Precision Immuno-Oncology often involves the use of combination therapies. This approach combines immunotherapy with targeted therapies, chemotherapy, radiation therapy, or other immune-modulating agents to enhance treatment efficacy and overcome resistance mechanisms. The global increase in cancer incidence, including various types of solid tumors and hematological malignancies, drives the demand for effective and precise oncology treatments like immuno-oncology.
The development of innovative immunotherapy approaches, such as immune checkpoint inhibitors, CAR-T cell therapy, and personalized vaccines, has significantly driven the precision immuno-oncology market. These therapies harness the immune system to target and eliminate cancer cells. The identification of specific biomarkers, such as PD-L1 expression levels, tumor mutational burden (TMB), and genetic mutations, has revolutionized cancer treatment. Biomarker testing helps in patient stratification and personalized treatment plans.
Advancements in genomics and molecular profiling technologies have allowed for a deeper understanding of the genetic and molecular characteristics of tumors. This knowledge informs treatment decisions and supports the development of targeted therapies. Patients are becoming more informed about cancer treatment options, including immunotherapy, through advocacy groups, online resources, and healthcare providers. This increased awareness can lead to greater patient demand for precision immuno-oncology.
Key Market Drivers
Rising Prevalence of Cancer
The rising prevalence of cancer worldwide is a significant driver propelling the growth of the Global Precision Immuno-Oncology Market. This factor creates a pressing demand for innovative, targeted, and personalized treatments. Cancer remains a leading cause of mortality globally. In 2022, nearly 20 million new cancer cases were reported globally, including nonmelanoma skin cancers (NMSCs), with approximately 9.7 million cancer-related deaths, also inclusive of NMSC. Data indicates that one in five individuals - male or female - is likely to develop cancer during their lifetime.Furthermore, cancer mortality rates reveal that one in nine men and one in twelve women succumb to the disease, highlighting its significant global impact and the urgent need for advanced therapeutic solutions. This sharp rise in cancer incidence underscores the urgent need for novel and effective therapies. Traditional approaches such as chemotherapy and radiation are often limited by non-specificity, significant side effects, and varying patient responses. Precision immuno-oncology therapies, by contrast, address these limitations by targeting specific biomarkers or genetic mutations unique to individual tumors.
Increased awareness campaigns and advancements in diagnostic technologies have led to higher detection rates of cancer. In 2022, lung cancer emerged as the most commonly diagnosed cancer globally, accounting for nearly 2.5 million new cases, or 12.4% of all cancer diagnoses. It was followed by female breast cancer (11.6%), colorectal cancer (9.6%), prostate cancer (7.3%), and stomach cancer (4.9%). Lung cancer also led in mortality, responsible for approximately 1.8 million deaths (18.7% of all cancer-related deaths), with colorectal (9.3%), liver (7.8%), female breast (6.9%), and stomach (6.8%) cancers following in order of mortality rates.
These figures underscore the disproportionate burden of lung cancer on global health and the critical need for targeted solutions in oncology. Early-stage detection is critical for precision immuno-oncology therapies, as these treatments are often more effective when administered early in disease progression. The availability of genomic testing and liquid biopsies has further enabled the identification of specific biomarkers, opening up opportunities for targeted immunotherapies. The heterogeneity of cancer has led to variability in patient responses to traditional treatments. This has driven demand for personalized medicine, a cornerstone of precision immuno-oncology.
These therapies are designed based on the molecular and genetic profile of a patient's tumor, ensuring better efficacy and minimizing adverse effects. For instance, immune checkpoint inhibitors (such as PD-1/PD-L1 inhibitors) and CAR-T cell therapies have shown remarkable success in treating cancers that were previously considered difficult to manage.
Many cancers develop resistance to standard treatments over time, leading to relapsed or refractory cases. This has created a critical need for advanced therapies that can overcome resistance mechanisms. Precision immuno-oncology therapies, which leverage the body’s immune system to attack cancer cells, offer a promising solution for these challenging cases. Their ability to adapt and target specific immune pathways makes them particularly effective against treatment-resistant cancers.
Cancer not only impacts patients and families but also imposes a significant economic burden on healthcare systems. The projected global economic cost of cancer from 2020 to 2050 is estimated at USD25.2 trillion in international dollars (adjusted to constant 2017 prices), representing an annual impact equivalent to 0.55% of the global GDP. The five cancers contributing most significantly to this economic burden are tracheal, bronchus, and lung cancer (15.4%); colorectal cancer (10.9%); breast cancer (7.7%); liver cancer (6.5%); and leukemia (6.3%). According to the American Cancer Society, global cancer-related healthcare costs exceed hundreds of billions of dollars annually.
Precision immuno-oncology therapies, while initially expensive, offer a cost-effective solution in the long run by improving survival rates, reducing hospitalization, and enhancing quality of life. This economic advantage is driving adoption among healthcare providers and insurers. Initially focused on a few cancer types (e.g., melanoma and lung cancer), precision immuno-oncology has expanded to treat a broad range of cancers, including breast, prostate, colorectal, and hematologic malignancies. The increasing prevalence of these cancers worldwide has amplified the need for diverse immuno-oncology solutions, leading to significant market growth.
Governments and organizations worldwide are prioritizing cancer research and treatment due to its growing prevalence. Initiatives such as the U.S. Cancer Moonshot and the European Cancer Plan are heavily funding research and clinical trials in immuno-oncology. These efforts are encouraging pharmaceutical companies to develop precision therapies tailored to the rising global cancer burden. The rising cancer prevalence has motivated pharmaceutical and biotech companies to allocate substantial R&D budgets toward immuno-oncology. According to industry reports, a significant portion of the oncology pipeline now focuses on precision-based immunotherapies.
The increasing volume of clinical trials and FDA/EMA approvals for immuno-oncology drugs underscores the demand created by the growing cancer burden. The rising prevalence of cancer is a critical market driver for precision immuno-oncology. By creating a demand for more effective, personalized, and targeted treatments, the global cancer burden is compelling stakeholders - governments, healthcare providers, pharmaceutical companies, and researchers - to invest heavily in this market. As cancer rates continue to climb, precision immuno-oncology is poised to play a central role in addressing this global health challenge, offering not only clinical efficacy but also economic and societal benefits.
Advancements in Immunotherapy
Immune checkpoint inhibitors (ICIs) have been a game-changer in cancer treatment. Drugs like pembrolizumab, nivolumab, and atezolizumab target proteins like PD-1 and PD-L1, allowing the immune system to recognize and attack cancer cells. They have shown remarkable success in various cancer types, including melanoma, lung cancer, and bladder cancer. Chimeric Antigen Receptor T-cell (CAR-T) therapy involves engineering a patient's T cells to express a receptor that targets cancer cells. CAR-T therapies like Kymriah and Yescarta have been approved for certain hematological malignancies and are being explored for other cancer types.Personalized cancer vaccines, like mRNA-based vaccines, are designed to stimulate the patient's immune system to target specific tumor antigens. These vaccines are tailored to the individual patient's genetic profile and tumor characteristics. Tumor-Infiltrating Lymphocytes (TILs) therapy involves extracting T cells from a patient's tumor, expanding them in the lab, and then reinfusing them back into the patient. This approach has shown promise in treating solid tumors.
Advances in biomarker identification, such as PD-L1 expression levels and microsatellite instability (MSI), enable oncologists to select patients who are more likely to respond to immunotherapy. This precision medicine approach optimizes treatment outcomes. Researchers are exploring combination therapies that incorporate immunotherapy with other treatments like targeted therapy, radiation therapy, and chemotherapy. Combinations aim to enhance efficacy while minimizing resistance.
Natural Killer (NK) cell therapies are emerging as a potential alternative to CAR-T cell therapy. NK cells can target cancer cells without the need for genetic modification, making them a versatile and promising immunotherapy approach. Ongoing research has led to a better understanding of immune-related adverse events (irAEs) associated with immunotherapy. Strategies for managing and reducing irAEs have improved patient safety. Liquid biopsies, which analyze circulating tumor DNA (ctDNA) and other biomarkers from a blood sample, allow for non-invasive monitoring of treatment response and the early detection of resistance.
AI-driven algorithms are being used to analyze large datasets, identify novel biomarkers, predict patient responses to treatment, and optimize treatment plans, contributing to the precision of immunotherapy. Clinical trials in immuno-oncology have evolved to incorporate biomarker-driven patient selection, adaptive trial designs, and real-world evidence, speeding up the development of new therapies. Neoantigens, unique to individual tumors, are being explored as targets for immunotherapy. Neoantigen vaccines and adoptive cell therapies are being developed to harness the immune system's ability to recognize these specific markers. This factor will help in the development of the Global Precision Immuno-Oncology Market.
Increasing Biomarker Discovery
Biomarkers help identify specific genetic, molecular, or protein characteristics of a patient's tumor. This information guides oncologists in selecting the most appropriate precision immuno-oncology therapy. Personalized treatment plans based on biomarkers can lead to more effective outcomes and better patient responses. Biomarker-driven therapies, such as immune checkpoint inhibitors and targeted therapies, have demonstrated higher response rates in patients whose tumors carry specific biomarkers. This increased efficacy encourages both healthcare providers and patients to seek precision immuno-oncology options.Biomarker-driven treatments are often associated with fewer side effects compared to traditional chemotherapy. This factor can improve patient quality of life and make precision immuno-oncology treatments more appealing. Biomarkers enable the design of clinical trials that target specific patient populations, increasing the likelihood of detecting treatment effects and expediting drug development. This attracts pharmaceutical companies and researchers to invest in precision immuno-oncology research.
By tailoring treatments based on biomarkers, healthcare systems can potentially reduce the costs associated with ineffective therapies and the management of treatment-related adverse events. This cost-effectiveness drives the adoption of precision immuno-oncology. Regulatory agencies, such as the FDA, often grant expedited approvals for therapies that demonstrate strong efficacy and safety profiles in biomarker-defined patient populations. This encourages pharmaceutical companies to develop and seek approval for precision immuno-oncology drugs. Patient advocacy groups and informed patients are increasingly advocating for biomarker testing and personalized treatment options.
Patients are more likely to seek out healthcare providers who offer precision immuno-oncology and biomarker-driven therapies. Biomarkers can identify patients who are unlikely to benefit from certain treatments. This information helps avoid overtreatment, reducing the physical and emotional burden on patients. Liquid biopsies and other biomarker-based tests allow for the early detection of cancer recurrence or treatment resistance. Regular monitoring using biomarkers can lead to timely treatment adjustments. Advances in genomics, proteomics, and molecular biology have expanded our understanding of cancer biology and the role of biomarkers. This knowledge has spurred the development of new biomarker-driven therapies. This factor will pace up the demand of the Global Precision Immuno-Oncology Market.
Rising Patient Awareness
Patients who are aware of precision immuno-oncology and its benefits are more likely to seek out information about these advanced treatments. Over 30% of cancer cases can be prevented through proactive lifestyle changes and the avoidance of key risk factors. Educating individuals about these risks is essential to reducing the global cancer burden. Major preventable risk factors include smoking, excessive alcohol consumption, poor diet, physical inactivity, obesity, and exposure to harmful infections such as HPV and Hepatitis B. They can make informed decisions about their treatment options, including requesting biomarker testing and personalized therapies. Informed patients often become advocates for their own health.They may actively engage with healthcare providers to explore precision immuno-oncology options and participate in shared decision-making regarding their treatment plans. Patients have access to a wealth of health information through the internet, patient advocacy organizations, and social media. This access allows them to educate themselves about the latest advancements in cancer treatment, including precision immuno-oncology.
Patient awareness can lead to increased demand for biomarker testing, such as PD-L1 expression testing or genomic profiling. Patients who understand the importance of these tests may request them to determine their eligibility for specific treatments. Informed patients may actively seek out clinical trial opportunities, especially those involving precision immuno-oncology. Their participation in trials can accelerate the development of new therapies and expand treatment options.
Patients who are aware of precision immuno-oncology may prioritize treatment options that offer better efficacy with fewer side effects. This consideration can lead to a preference for precision therapies over traditional treatments like chemotherapy. Informed patients may seek second opinions from oncologists or specialized cancer centers to explore the full range of precision immuno-oncology options available to them. Online and in-person patient support communities and advocacy groups provide a platform for patients to share their experiences and knowledge about precision immuno-oncology. These communities can further raise awareness and empower patients to advocate for their care.
Patients who are informed about precision immuno-oncology may feel more empowered to actively engage in their treatment decisions, ask questions, and discuss treatment options with their healthcare providers. Healthcare providers and advocacy organizations are investing in patient education materials and initiatives to ensure that patients have access to accurate and up-to-date information about precision immuno-oncology. Increased awareness of precision immuno-oncology can help reduce the stigma associated with cancer and its treatment. This can encourage more patients to seek timely medical care and explore advanced treatment options. This factor will accelerate the demand of the Global Precision Immuno-Oncology Market.
Key Market Challenges
Tumor Heterogeneity
Tumor heterogeneity refers to the presence of diverse cell populations within a single tumor. These populations may have distinct molecular profiles, genetic mutations, and immune characteristics. This diversity makes it challenging to identify a single, effective targeted therapy or immunotherapy that can address all aspects of the tumor. Heterogeneous tumors can develop resistance to therapies. While a treatment may initially target and eliminate some tumor cell populations, it may not be effective against others. This can lead to partial responses, disease recurrence, or the emergence of treatment-resistant clones within the tumor.Tumor heterogeneity can result in variations in biomarker expression within the same tumor. For example, the level of PD-L1 expression or the presence of specific genetic mutations may vary across different regions of the tumor. This complicates the selection of patients for precision immuno-oncology treatments based on biomarker testing. Identifying the most therapeutically relevant target within a heterogeneous tumor can be challenging. Some subpopulations of cells may carry driver mutations or immune evasion mechanisms that are more critical for treatment decisions than others.
Resistance Mechanisms
Tumors can adapt and develop resistance to immunotherapy and targeted therapies over time. This acquired resistance can lead to treatment failure and disease progression, reducing the long-term benefits of these therapies. Resistance mechanisms can vary among different tumor subpopulations, leading to heterogeneous responses within the same tumor. This heterogeneity complicates treatment decisions and may require combination therapies or tailored approaches. Tumors can acquire new mutations that render them resistant to therapy. These mutations may affect the target of the therapy or involve alternative signaling pathways, making it challenging to predict and overcome resistance.Tumors can create an immunosuppressive microenvironment that inhibits the immune system's ability to attack cancer cells. This microenvironment may involve the recruitment of regulatory T cells, myeloid-derived suppressor cells, and the upregulation of immune checkpoint proteins like PD-L1. Some tumors may downregulate or lose expression of the target antigen or protein that the therapy is designed to target. This can render the therapy ineffective, as there is no longer a specific target for the treatment.
Tumors can alter the way they present antigens to immune cells, making it difficult for the immune system to recognize and attack cancer cells effectively. This can lead to immune evasion and resistance. Changes in the tumor microenvironment, including increased fibrosis, hypoxia, and nutrient deprivation, can create a hostile environment for immune cells, hindering their ability to infiltrate and attack the tumor. Some tumors may upregulate secondary immune checkpoints in response to treatment with immune checkpoint inhibitors, providing alternative inhibitory signals to immune cells.
Key Market Trends
Patient-Centric Approaches
Patient-centric care in precision immuno-oncology emphasizes personalized treatment plans tailored to the individual patient's unique characteristics. This includes considering the patient's biomarker profile, genomic data, and treatment preferences. Healthcare providers are increasingly engaging patients in shared decision-making. Patients are informed about their treatment options, including the benefits and potential risks, and are encouraged to actively participate in choosing the most suitable therapy. Providing patients with accessible and understandable information about their disease, treatment options, and potential side effects is a crucial aspect of patient-centric care.Education empowers patients to make informed choices and adhere to treatment plans. Improving patients' quality of life is a central goal. Precision immuno-oncology treatments are designed not only to extend survival but also to enhance patients' well-being and maintain or improve their functional status. Patient-centric approaches prioritize the proactive management of treatment-related side effects. Oncology teams work closely with patients to monitor and address side effects, ensuring that treatment is as tolerable as possible.
Segmental Insights
Treatment Type Insights
In 2024, the Global Precision Immuno-Oncology Market largest share was held by checkpoint inhibitors segment and is predicted to continue expanding over the coming years. Immune checkpoint inhibitors, such as drugs targeting PD-1 and PD-L1, have demonstrated remarkable clinical success in various cancer types. They have shown the ability to induce durable responses and improve survival rates in some patients. Immune checkpoint inhibitors have shown effectiveness across multiple cancer types, including melanoma, lung cancer, bladder cancer, and more.This broad applicability has made them a cornerstone of precision immuno-oncology treatments. The use of biomarkers, such as PD-L1 expression levels and microsatellite instability (MSI), to select patients who are more likely to respond to immune checkpoint inhibitors has become a standard practice. This precision in patient selection enhances the overall effectiveness of these therapies. Leading cancer treatment guidelines, such as those from the National Comprehensive Cancer Network (NCCN) and the American Society of Clinical Oncology (ASCO), recommend the use of immune checkpoint inhibitors in various cancer settings.
Cancer Type Insights
In 2024, the Global Precision Immuno-Oncology Market largest share was held by lung cancer segment and is predicted to continue expanding over the coming years. Lung cancer is one of the most diagnosed cancers globally, and it has a significant impact on public health. The high incidence of lung cancer contributes to the attention and resources dedicated to developing precision immuno-oncology treatments for this disease. Biomarker testing, including PD-L1 expression levels and tumor mutational burden (TMB), is commonly used to identify lung cancer patients who are more likely to respond to immunotherapy. This precision medicine approach ensures that the right patients receive the most suitable treatment. Increased awareness among patients and healthcare providers, as well as patient advocacy efforts, have contributed to the demand for and utilization of immunotherapy in lung cancer treatment.End-User Insights
In 2024, the Global Precision Immuno-Oncology Market largest share was held by Hospitals & Clinics segment in the forecast period and is predicted to continue expanding over the coming years. Hospitals and clinics are primary healthcare providers where patients receive a wide range of medical services, including cancer diagnosis and treatment. They serve as hubs for precision immuno-oncology services, such as biomarker testing, genetic profiling, and personalized treatment planning. Patients diagnosed with cancer are often referred to hospitals and clinics by primary care physicians or other healthcare professionals.These institutions have the infrastructure and expertise to conduct further assessments and deliver specialized immuno-oncology care. Hospitals and clinics typically have multidisciplinary teams of oncologists, pathologists, radiologists, and genetic counsellors who collaborate to provide comprehensive precision immuno-oncology services. This team approach is crucial for tailoring treatment plans to individual patients. Many precisions immuno-oncology clinical trials are conducted in collaboration with hospitals and clinics. These institutions have access to a diverse patient population, making them ideal locations for enrolling patients in clinical research studies.
Regional Insights
The North America region dominates the Global Precision Immuno-Oncology Market in 2024. North America, particularly the United States and Canada, boasts advanced healthcare infrastructure, including top-tier medical institutions, research centers, and hospitals. These institutions have the resources and expertise to lead in the development and adoption of precision immuno-oncology. The region is home to many pharmaceutical and biotechnology companies that are at the forefront of immuno-oncology research and development.They invest heavily in the discovery and commercialization of precision therapies. North America has a robust venture capital and private equity ecosystem, which provides substantial funding for startups and innovative companies working on precision immuno-oncology solutions. North America conducts a significant portion of global clinical trials, attracting patients from around the world to participate in research studies. This helps in the advancement and validation of precision immuno-oncology therapies.
Key Market Players
- Adaptimmune Therapeutics Plc
- Agenus Inc.
- Amgen Inc
- AstraZeneca plc
- Bristol Myers Squibb Company
- Celgene Corporation
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc
- GlaxoSmithKline Plc
Report Scope:
In this report, the Global Precision Immuno-Oncology Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Precision Immuno-Oncology Market, By Cancer Type:
- Lung cancer
- Breast Cancer
- Prostate Cancer
- Melanoma
- Others
Precision Immuno-Oncology Market, By Treatment Type:
- Lung cancer
- Breast Cancer
- Prostate Cancer
- Melanoma
- Others
Precision Immuno-Oncology Market, By Biomarker:
- PD-L1
- BRCA
- KRAS
- EGFR
- Others
Precision Immuno-Oncology Market, By End-User:
- Hospital & Clinics
- Research Institutes
- Others
Precision Immuno-Oncology Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Precision Immuno-Oncology Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Adaptimmune Therapeutics Plc
- Agenus Inc.
- Amgen Inc
- AstraZeneca plc
- Bristol Myers Squibb Company
- Celgene Corporation
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences, Inc
- GlaxoSmithKline Plc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2025 |
| Forecast Period | 2024 - 2030 |
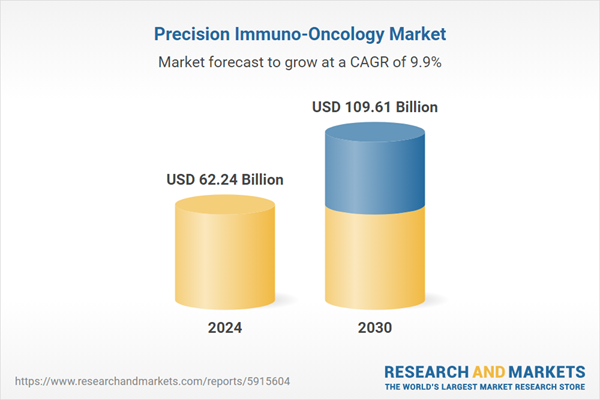
| Estimated Market Value ( USD | $ 62.24 Billion |
| Forecasted Market Value ( USD | $ 109.61 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |