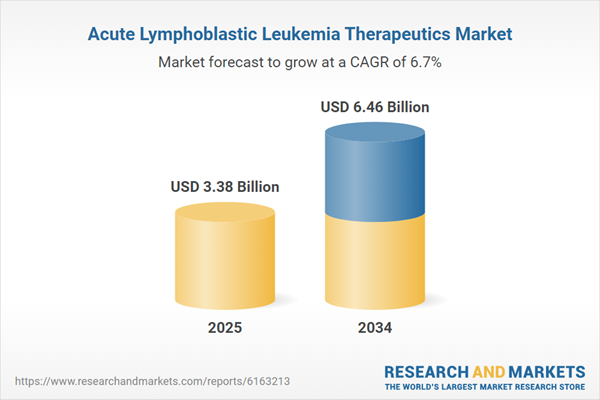
Acute Lymphoblastic Leukemia Therapeutics Market Overview
Acute lymphoblastic leukemia (ALL) is a cancer of blood and bone marrow, specifically affecting the lymphocytes (white blood cells). It is the most common type of cancer in children but can also occur in adults. It accounts for 74% of the overall pediatric leukemia cases. The risk of developing the disease gradually declines until the mid-20s and begins to rise after a person attains the age of 50. ALL affects approximately 4 out of every 10 adults. While most cases affect children, the death rate is significantly higher in adults (about 4 out of 5).Acute lymphoblastic leukemia therapeutics market demand is driven by the rising incidence of the disease in recent years. As per data released by the American Cancer Society in 2023, around 6,540 new ALL cases are expected to affect the United States population, out of which 3,660 cases are expected in males and 2,880 cases in females. The deaths associated with the disease are projected to be around 1,390 (700 in males and 690 in females). While it is not a common cancer, the average person's lifetime risk of developing the disease is 1 in 1,000. The male community is more susceptible to this category of tumor than the females.
Surge in Combination Therapies for Personalized Treatment
The acute lymphoblastic leukemia therapeutics market share is expected to be dominated by combination therapies, owing to their efficacy in reducing tumor progression and minimizing other side effects associated with the condition. In January 2023, the results of a clinical trial revealed that blinatumomab (Blincyto), when combined with chemotherapy, showed 83% improvement in the survival rate as opposed to those who were subjected to chemotherapy alone (65%).Growth in Immunotherapy and Targeted Therapies to Drive the Market Growth
With the rising popularity of targeted therapy to treat cancer, a class of tyrosine kinase inhibitors (TKI) has been found effective in treating newly diagnosed ALL in patients. One such example is Takeda Pharmaceutical's Ponatinib, which is currently under phase I trial for pediatric Ph+ acute lymphoblastic leukemia. It prevents tumor progression by blocking BCR-ABL protein.Asparaginase therapies are also considered as critical treatment alternative for acute lymphoblastic leukemia. Currently, the FDA has approved five asparaginase therapies. Owing to its reduced immunogenicity compared to native E. coli preparations and satisfactory pharmacokinetic profile, pegaspargase is used as the firsthand asparaginase therapeutic option. To meet the rising acute lymphoblastic leukemia therapeutics market demand, regulatory authorities around the world are approving new drug therapies for treatment.
In July 2023, the European Medicines Agency (EMA) showed a positive opinion on the authorization of marketing Jazz Pharmaceutical's JZP458 (a recombinant Erwinia asparaginase or crisantaspase) in the region. L -asparaginase lowers the serum levels of L-asparagine, resulting in cell death. This is because the enzyme is present in the human body and not naturally synthesized by the leukemic cells. It is expected to be used as a constituent of a multi-agent chemotherapeutic routine for the treatment of acute lymphoblastic leukemia in adults as well as children who have developed silent inactivation or hypersensitivity to E. coli derived asparaginase.
Emphasis on Cancer Recurrence
Preventing recurrence of acute lymphoblastic leukemia is a key area of investigation amongst scientists. Hence, there is an emphasis on pairing standard leukemia treatments with new class of drugs to prevent relapse. In addition, immune checkpoint blockade that disrupts the pathway used by cancer cells to evade the immune system are also being evaluated in clinical trials. The search for new technologies and alternatives, coupled with improving technical advancements, is expected to support the acute lymphoblastic leukemia therapeutics market size in the forecast period.Acute Lymphoblastic Leukemia Therapeutics Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- B-cell Acute Lymphoblastic Leukemia
- C-cell Acute Lymphoblastic Leukemia
Market Breakup by Treatment
- Chemotherapy
- Radiation Therapy
- Bone Marrow Transplant
- Targeted Therapy
- Immunotherapy
- Others
Market Breakup Patient Type
- Adult
- Pediatric
Market Breakup by Route of Administration
- Oral
- Parenteral
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Market Breakup by Region
- United States
- United Kingdom
- Germany
- France
- Italy
- Spain
- Japan
- India
Acute Lymphoblastic Leukemia Therapeutics Market Regional Analysis
The United States, with a well-established medical and research infrastructure is expected to lead the acute lymphoblastic leukemia therapeutics market share in upcoming years. The market size is further fueled by the inauguration of research centers with a vision to encourage research and innovation. In September 2023, Cincinnati Children's Hospital launched advanced leukemia therapies and research center that aims to integrate more research and clinical programs for improved patient outcomes.Acute Lymphoblastic Leukemia Therapeutics Market: Competitor Landscape
In December 2023, Theseus Pharmaceuticals entered a merger agreement with Concentra Biosciences wherein the company will acquire Theseus Pharmaceuticals for a stock price of USD 3.90 and USD 4.05 in cash. The merger is focused on improving the lives of cancer patients with discovery, commercialization, and development of transformative targeted therapies.In March 2023, Pfizer acquired Seagen for USD 43 billion to develop antibody drug candidates, a pioneer in the cancer class drugs. Such acquisitions are a clear indicator that the market for cancer therapeutics is anticipated to witness substantial growth in the forecast period.
The key features of the acute lymphoblastic leukemia therapeutics market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:
- Novartis AG
- F. Hoffmann-La Roche Ltd
- Eisai Co Ltd
- GlaxoSmithKline plc.
- Celegene Corporation
- Bristol Myer Squibb Company
- Pfizer Inc.
- Sanofi SA
- Genmab A/S
- Erytech Pharma
- Takeda Pharmaceutical Company Limited
- OBI Pharma
- Astellas Pharma Inc.
- Medexus Pharma, Inc.
- Kiadis Pharma
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Novartis AG
- F. Hoffmann-La Roche Ltd
- Eisai Co Ltd
- GlaxoSmithKline plc.
- Celegene Corporation
- Bristol Myer Squibb Company
- Pfizer Inc.
- Sanofi SA
- Genmab A/S
- Erytech Pharma
- Takeda Pharmaceutical Company Limited
- OBI Pharma
- Astellas Pharma Inc.
- Medexus Pharma, Inc.
- Kiadis Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 3.38 Billion |
| Forecasted Market Value ( USD | $ 6.46 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |