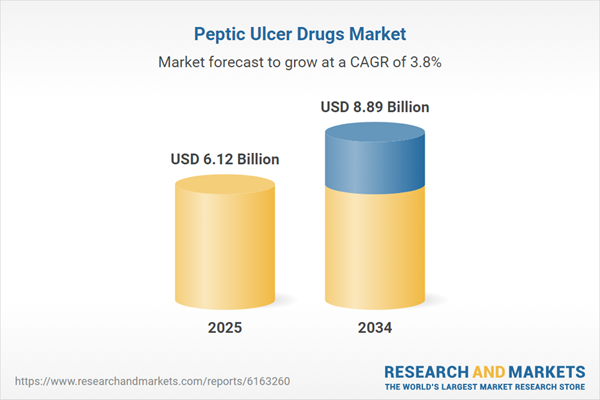
Peptic Ulcer Drugs Market Overview
Peptic ulcers are characterized by open sores on the lining of the esophagus, stomach, or small intestine. The most common symptom is pain in the upper abdominal region. It is usually caused by the bacterium Helicobacter pylori and long-term use of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin. Peptic ulcers affect 1 out of 12 individuals in the United States with 1 in 5 cases associated with the bacterial infection . The rising prevalence of the disease is one of the key factors driving the peptic ulcer drugs market demand and is anticipated to accelerate the growth of the market in the forecast period.The surge in drug approvals by the health regulatory bodies is expected to further bolster the market growth. In October 2023, the United States Food and Drug Administration (FDA) approved reformulated vonoprazan tablets for Voquezna Triple Pak and Voquezna Dual Pak to treat Helicobacter pylori infection in adults. Developed by Phathom Pharmaceuticals, a United States-based biopharmaceutical company, these drugs combine antibiotics with vonoprazan, a novel potassium-competitive acid blocker. This new class of acid suppression therapy offers promising H. pylori eradication rates. The company plans to commercially launch it in December 2023 for H. pylori treatment.
The peptic ulcer drugs market share is also expected to experience a significant expansion due to increasing research and development activities backed by robust support from the government. Moreover, the growing geriatric population and advancement in the healthcare system will also boost the market growth.
Peptic Ulcer Drugs Market Segmentation
Peptic Ulcer Drugs Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Product Type
- Proton Pump Inhibitor
- H2 Antagonist
- Antibiotics
- Antacids
- Ulcer Protective
Market Breakup by Disease Indication
- Gastritis
- Gastric Ulcer
- Duodenal Ulcer
- GERD
- Others
Market Breakup by Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Germany
- France
- Italy
- Spain
- United Kingdom
- Japan
- India
Peptic Ulcer Drugs Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Orion Corporation
- Bausch Health Companies Inc.
- CYTOKINETICS, INC.
- Aquestive Therapeutics, Inc.
- Sanofi
- Covis Pharma
- Sun Pharmaceuticals Industries Ltd
- Mitsubishi Tanabe Pharma Corporation
- BrainStorm Cell Limited
- ViroMed Co., Ltd
- Ionis Pharmaceuticals
- Genervon Biopharmaceuticals, LLC
- Biogen
- Orphazyme A/S
- F. Hoffmann-La Roche Ltd
Key Queries Solved in the Peptic Ulcer Drugs Market Report
- How is the market landscape expected to evolve in coming years?
- What are the major market trends influencing the market?
- What are the major drivers, opportunities, and restraints in the market?
- What will be the effect of each driver, challenge, and opportunity on the market?
- Which country is poised to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What factors contribute to the rising peptic ulcers market demand?
- What is the incidence and epidemiology of peptic ulcers?
- What are the different types of peptic ulcer drugs available in the market?
- What are the latest advancements in peptic ulcer drug research and development, including novel drug candidates and formulations?
- Which drugs are under clinical trials?
- What were the major drug approvals used to manage peptic ulcers during the historical period?
- Which major drug manufacturers are expected to lead the market?
- What are the key strategies adopted by leading pharmaceutical companies to gain market share?
- How are partnerships, collaborations, and mergers & acquisitions shaping the market dynamics of peptic ulcer drugs?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Orion Corporation
- Bausch Health Companies Inc.
- CYTOKINETICS, INC.
- Aquestive Therapeutics, Inc.
- Sanofi
- Covis Pharma
- Sun Pharmaceuticals Industries Ltd
- Mitsubishi Tanabe Pharma Corporation
- BrainStorm Cell Limited
- ViroMed Co., Ltd
- Ionis Pharmaceuticals
- Genervon Biopharmaceuticals, LLC
- Biogen
- Orphazyme A/S
- F. Hoffmann-La Roche Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 6.12 Billion |
| Forecasted Market Value ( USD | $ 8.89 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |