Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Conversely, a significant barrier to market progression is the high frequency of misdiagnosis due to substantial symptomatic overlap with Alzheimer’s and Parkinson’s diseases. This diagnostic ambiguity often delays appropriate care and restricts the effective deployment of specific treatments. As reported by the Alzheimer's Association in 2024, approximately 5% of older individuals with dementia show evidence of Lewy body dementia alone, a statistic that underscores the complex challenge of isolating this specific patient segment from those with mixed pathologies within the broader dementia population.
Market Drivers
A robust clinical pipeline and accelerated drug development efforts serve as a primary catalyst for market expansion, driven by the urgent need for disease-modifying therapies rather than mere symptomatic relief. Pharmaceutical companies are increasingly targeting novel mechanisms of action, such as p38 alpha kinase inhibition, to address the underlying synaptic dysfunction associated with Lewy bodies. This intensification of late-stage clinical trials is bringing potential treatments closer to regulatory approval, offering hope for a patient population that has historically relied on off-label medications. For example, CervoMed reported in October 2024 the completion of the final patient visit in its RewinD-LB Phase 2b clinical trial for neflamapimod, marking a critical milestone in advancing specific therapeutics for this indication.Market growth is further solidified by increasing public and private investment in neurological research, which provides the necessary capital for high-risk, high-reward discovery programs. Enhanced funding supports large-scale longitudinal studies and biomarker validation, which are essential for stratifying patient populations and improving trial success rates. A notable instance occurred in September 2024, when Penn Medicine announced that the National Institute on Aging awarded an $18 million grant to researchers to investigate the causes of cognitive decline and identify biomarkers in Lewy body diseases. These investments are crucial given the substantial economic burden the disease places on healthcare systems, incentivizing payers and providers to seek effective treatments; a systematic review in BJPsych Open in January 2024 highlighted that average medical costs rose to $29,174 in the first year following an LBD diagnosis, underscoring the immense financial value of developing effective clinical solutions.
Market Challenges
The primary obstacle hindering the growth of the Global Lewy Body Dementia Treatment Market is the high rate of misdiagnosis caused by symptomatic overlaps with Alzheimer’s and Parkinson’s diseases. This diagnostic ambiguity significantly restricts the addressable market, as a large proportion of patients are treated for other neurodegenerative conditions rather than receiving therapies specifically optimized for Lewy body dementia. Consequently, pharmaceutical developers face substantial hurdles in identifying suitable patient cohorts for clinical trials, which delays the approval and commercialization of targeted treatments. The inability to accurately segregate this patient population reduces the perceived return on investment for drug manufacturers, thereby stalling R&D progress and limiting market expansion.This issue effectively conceals a massive segment of the potential customer base from market participants. According to the Lewy Body Dementia Association, an estimated 1.4 million individuals in the United States were affected by Lewy body dementia in 2024. This statistic reveals a profound gap between the actual prevalence of the disease and the number of accurately diagnosed cases. As long as these individuals remain unidentified or misclassified within the broader dementia population, the market cannot fully capitalize on the demand for specific Lewy body dementia interventions, directly impeding overall revenue potential.
Market Trends
The integration of cutaneous synuclein biomarkers for early diagnosis represents a critical evolution in market practice, shifting the sector from a reliance on clinical observation toward objective pathological validation in living patients. This trend resolves the historic difficulty of confirming alpha-synuclein deposition without post-mortem analysis, thereby enabling more accurate patient identification for clinical trials and commercial treatment. In May 2024, the Lewy Body Dementia Association reported that the NIH-funded Synuclein-One Study demonstrated that a cutaneous phosphorylated alpha-synuclein test achieved a 96% positive detection rate in participants clinically diagnosed with Lewy body dementia. Such diagnostic precision is essential for accurately segregating these patients from those with pure Alzheimer’s disease, ensuring that emerging targeted therapies reach the correct demographic.Simultaneously, the market is witnessing a decisive shift toward disease-modifying alpha-synuclein immunotherapies, which aim to halt neurodegeneration by clearing toxic protein aggregates rather than merely managing symptoms. Developers are prioritizing active vaccination strategies that train the patient's immune system to recognize and eliminate pathological oligomers before they cause irreversible neuronal damage. Validating this approach, AC Immune announced in November 2024 that interim results from the Phase 2 VacSYn trial showed the active immunotherapy candidate ACI-7104.056 induced anti-alpha-synuclein antibody levels on average 16-fold higher than placebo after three doses. This mechanism suggests a potential for slowing disease progression, fundamentally altering the long-term treatment landscape for affected individuals.
Key Players Profiled in the Lewy Body Dementia Treatment Market
- BioArctic AB
- Eisai Co., Ltd.
- Sumitomo Pharma Co., Ltd.
- Jazz Pharmaceuticals, Inc.
- Immungenetics ANoven Pharmaceuticals, Inc.
- Eli Lilly and Company
- Novartis AG
- Pfizer Inc.
- Mylan N.V.
Report Scope
In this report, the Global Lewy Body Dementia Treatment Market has been segmented into the following categories:Lewy Body Dementia Treatment Market, by Distribution Channel:
- Hospital Pharmacies
- Retail Pharmacies
- Drug Stores
- Online Pharmacies
Lewy Body Dementia Treatment Market, by Drug Type:
- Cholinesterase Inhibitors
- Antipsychotic Drugs
- Carbidopa-Levodopa
- Antidepressants
- Benzodiazepine
- Modafinil
Lewy Body Dementia Treatment Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Lewy Body Dementia Treatment Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Lewy Body Dementia Treatment market report include:- BioArctic AB
- Eisai Co., Ltd
- Sumitomo Pharma Co., Ltd.
- Jazz Pharmaceuticals, Inc.
- Immungenetics ANoven Pharmaceuticals, Inc.
- Eli Lilly and Company
- Novartis AG
- Pfizer Inc.
- Mylan N.V.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
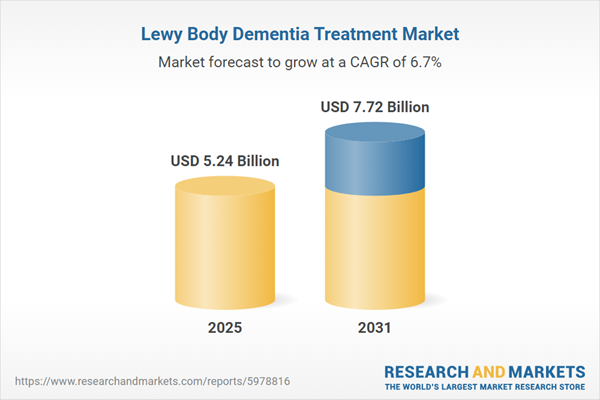
| Estimated Market Value ( USD | $ 5.24 Billion |
| Forecasted Market Value ( USD | $ 7.72 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |