Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
However, the market faces a substantial obstacle in the form of the prohibitive costs attached to sophisticated treatment modalities. The steep pricing of novel immunotherapies, including CAR-T cell treatments, engenders significant reimbursement challenges and restricts patient access, especially in areas with limited healthcare funding. When combined with the intricate manufacturing processes required for autologous therapies, these economic barriers hinder the widespread uptake of life-saving treatments and constrain the broader expansion of the global market.
Market Drivers
The Global Acute Lymphocytic Leukemia Therapeutics Market is being fundamentally transformed by the rapid adoption of CAR-T cell therapies and innovative immunotherapies, which offer durable remission for patients with relapsed or refractory conditions. This transition from traditional chemotherapy to immune-engaging strategies is highlighted by the strong commercial performance of bispecific T-cell engagers, now emerging as standard-of-care options. For example, Amgen reported in its 'Third Quarter 2024 Financial Results' in October 2024 that sales of its immunotherapy Blincyto surged by 49% year-over-year to reach $327 million, a growth attributed largely to increased volume across clinical settings. These trends illustrate the medical community's deepening reliance on targeted immune mechanisms to enhance survival rates in high-risk groups, confirming the significant value of these premium biologics.Concurrently, the market is growing due to a wave of regulatory approvals for next-generation targeted therapeutics, continuously expanding the range of available treatment options. Regulatory bodies are increasingly authorizing advanced biologics that show superior efficacy in key trials, which in turn stimulates further investment and clinical development. A notable instance occurred in November 2024, when Autolus Therapeutics announced FDA approval for AUCATZYL, a CAR-T therapy that achieved a 42% complete remission rate within three months for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. This regulatory progress is supported by the consistent revenue of established therapies; Gilead Sciences reported in November 2024 that Tecartus generated $98 million in sales for the third quarter, driven largely by its use in treating adult leukemia.
Market Challenges
A significant impediment to the Global Acute Lymphocytic Leukemia Therapeutics Market is the exorbitant cost associated with advanced pharmacological treatments. The steep price tags of novel immunotherapies, particularly CAR-T cell treatments, establish substantial economic barriers that prevent broad market penetration. Because these sophisticated modalities necessitate specialized administration and intricate manufacturing procedures, their pricing structures place a heavy strain on healthcare budgets and complicate reimbursement processes. As a result, patient access is severely curtailed, especially in regions with limited financial resources, directly undermining the potential market growth that clinical efficacy would otherwise drive.The severity of this financial burden is highlighted by the current cost landscape of these therapies. According to data from the Association of Community Cancer Centers, the acquisition cost for CAR T-cell therapy products in 2025 fell between $373,000 and $475,000, with the total aggregate cost of patient care frequently surpassing $1 million. Such immense expenses pose a formidable challenge for both healthcare providers and payers, leading to delays in treatment authorization and limiting the commercial scalability of these essential innovations. Consequently, this economic friction persists as a critical constraint on the overall growth trajectory of the global therapeutics market.
Market Trends
A major trend in the market is the paradigmatic shift toward chemotherapy-free therapeutic regimens, specifically for patients with Philadelphia chromosome-positive Acute Lymphocytic Leukemia. Clinicians are increasingly adopting combinations of next-generation tyrosine kinase inhibitors and bispecific T-cell engagers to achieve deep molecular responses while avoiding the systemic toxicity typical of intensive chemotherapy. This approach reduces the physiological burden on patients and has shown superior efficacy in sustaining long-term remission, challenging the traditional reliance on transplant-based standards. Highlighting the potential of this strategy, The ASCO Post reported in August 2024 that an updated analysis of a chemotherapy-free regimen combining ponatinib and blinatumomab yielded a complete molecular response rate of 87% in adults, underscoring the clinical utility of this sparing approach.In parallel, the integration of Minimal Residual Disease (MRD) testing is becoming central to treatment protocols, fundamentally reshaping patient management. High-sensitivity Next-Generation Sequencing assays are superseding traditional morphological assessments, enabling providers to identify sub-microscopic disease burdens and adjust interventions based on the genetic depth of remission. This move toward precision medicine facilitates the use of advanced diagnostics to monitor treatment efficacy and predict relapse risks earlier than before. Reflecting this growing reliance on detailed diagnostic data, Adaptive Biotechnologies reported in November 2024 that clinical test volumes for their clonoSEQ MRD assay rose by 30% year-over-year to 19,600 tests, driven by widespread adoption across hematological indications.
Key Players Profiled in the Acute Lymphocytic Leukemia Therapeutics Market
- Takeda Pharmaceutical Company Limited.
- SymBio Pharmaceuticals Limited
- Pfizer, Inc.
- Jazz Pharmaceuticals, Inc.
- Sanofi AG
- Amgen, Inc.
- Bristol-Myers Squibb Company
- Leadiant Biosciences, Inc.
- Novartis AG
- Rare Disease Therapeutics, Inc.
Report Scope
In this report, the Global Acute Lymphocytic Leukemia Therapeutics Market has been segmented into the following categories:Acute Lymphocytic Leukemia Therapeutics Market, by Therapy:
- Chemotherapy
- Hyper-CVAD
- CALGB 8811 regimen
- Linker regimen
- Nucleoside Metabolic Inhibitors
- Oncaspar
- Targeted therapy
- Radiation Therapy
- Stem cell transplantation
Acute Lymphocytic Leukemia Therapeutics Market, by Type:
- Philadelphia chromosome
- Precursor B-cell ALL
- T-cell ALL
Acute Lymphocytic Leukemia Therapeutics Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Acute Lymphocytic Leukemia Therapeutics Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Acute Lymphocytic Leukemia Therapeutics market report include:- Takeda Pharmaceutical Company Limited.
- SymBio Pharmaceuticals Limited
- Pfizer, Inc.
- Jazz Pharmaceuticals, Inc.
- Sanofi AG
- Amgen, Inc.
- Bristol-Myers Squibb Company
- Leadiant Biosciences, Inc.
- Novartis AG
- Rare Disease Therapeutics, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
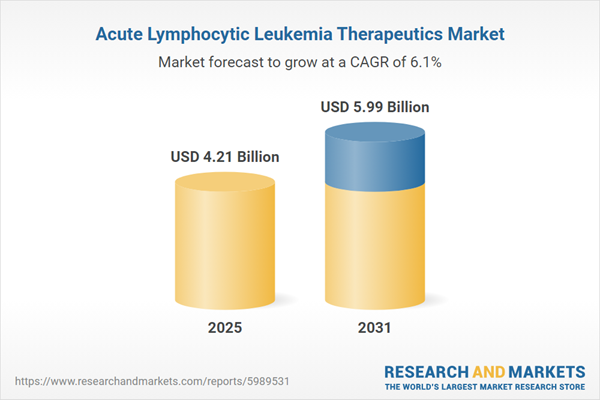
| Estimated Market Value ( USD | $ 4.21 Billion |
| Forecasted Market Value ( USD | $ 5.99 Billion |
| Compound Annual Growth Rate | 6.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |