Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Despite this strong demand, the market encounters significant hurdles related to the accuracy and interpretation of home-based test results. Procedural errors by users or physiological limitations often result in false readings, fostering consumer skepticism and leading to unnecessary medical consultations. This issue of reliability is frequently compounded by rigorous regulatory environments in emerging economies, which often delay the approval of enhanced testing formats and limit the general population's access to dependable diagnostic devices.
Market Drivers
Technological innovations in digital and connected diagnostics are transforming the Global Pregnancy Testing Devices Market by satisfying consumer preferences for hygiene and simplicity. Novel methods, such as saliva-based testing, are rapidly gaining traction as non-invasive alternatives that eliminate the discomfort associated with traditional urine strips. For instance, a Forbes article from December 2024 noted that Salignostics sold over 500,000 units of its saliva-based test shortly after launch, signaling a decisive shift toward user-centric product designs. Furthermore, the economic scale of this segment is widening; a November 2025 report from Femtech Insider highlighted that Germany’s self-testing market alone generated approximately $508 million in 2024, demonstrating the robust financial potential of private health monitoring tools.Concurrently, the rising prevalence of infertility and the trend of delayed parenthood are shifting market dynamics from reactive confirmation to proactive fertility management. As women increasingly postpone childbirth, the biological window for conception narrows, requiring frequent and precise testing to monitor reproductive cycles. According to provisional data released by the Centers for Disease Control and Prevention in July 2025, the U.S. general fertility rate fell to 53.8 births per 1,000 females in 2024. Paradoxically, this decline stimulates market growth, as the difficulty in conceiving compels couples to rely more heavily on diagnostic devices for assisted reproduction protocols and rigorous family planning strategies.
Market Challenges
A major obstacle impeding the Global Pregnancy Testing Devices Market is the challenge of ensuring accuracy and correct interpretation of home-based results. User procedural errors, such as improper timing or sample collection, frequently yield false readings that erode consumer confidence. When individuals cannot trust the reliability of a home test, they are less likely to rely on these devices for immediate confirmation, often turning to professional medical intervention instead. This consumer distrust creates a significant barrier to market penetration, particularly for new product adoption, as the fear of misdiagnosis often outweighs the convenience offered by at-home testing.This challenge is further intensified by stringent regulatory frameworks which, while designed to guarantee safety, create substantial bottlenecks for market growth. Regulatory bodies in both emerging and developed economies are enforcing stricter clinical evidence requirements, slowing the approval process for improved, more user-friendly testing formats. According to MedTech Europe, in 2024, 30% of In Vitro Diagnostic manufacturers reported significant delays or negative outcomes regarding device certification due to rigorous performance evaluations. These delays prevent manufacturers from swiftly introducing advanced solutions that could mitigate user errors, thereby stalling the market's ability to evolve and meet consumer demand for reliability.
Market Trends
The industry is witnessing a significant shift toward the adoption of biodegradable and flushable materials, driven by escalating consumer demand for environmentally responsible diagnostic solutions. Manufacturers are increasingly prioritizing plastic-neutral designs to mitigate the substantial waste generated by single-use diagnostic kits, moving away from traditional non-recyclable casings. This transition is reshaping product development pipelines as companies seek to align with global sustainability goals without compromising diagnostic accuracy. As evidence of this commercial focus, Abingdon Health’s March 2025 interim results reported H1 2025 revenues of £3.1 million and confirmed the upcoming U.S. launch of its sustainable pregnancy tests, underscoring the prioritization of eco-friendly innovations.Simultaneously, the expansion of direct-to-consumer (DTC) and online subscription models is revolutionizing market access by addressing the consumer need for privacy and logistical convenience. Unlike traditional retail purchasing, these digital-first channels offer discreet, recurring deliveries that allow individuals to manage reproductive health monitoring from home, bypassing the potential discomfort of pharmacy visits. This distribution shift is supported by robust digital engagement; according to Church & Dwight Co., Inc.'s January 2025 earnings call, online sales accounted for 21.4% of their total global sales in 2024, reflecting a significant migration of consumer purchasing behavior toward digital platforms for personal care products.
Key Players Profiled in the Pregnancy Testing Devices Market
- Abbott
- Roche
- BD
- Siemens Healthineers
- Beckman Coulter
- Acon Laboratories
- SD Biosensor
- Hologic
- AmniSure
- Quest Diagnostics
Report Scope
In this report, the Global Pregnancy Testing Devices Market has been segmented into the following categories:Pregnancy Testing Devices Market, by Product Type:
- Urine based
- Blood based
Pregnancy Testing Devices Market, by Technology:
- Line Indicator
- Digital Test
Pregnancy Testing Devices Market, by Distribution Channel:
- Retail Pharmacies
- Online Pharmacies
- Hospital Pharmacies
Pregnancy Testing Devices Market, by Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Pregnancy Testing Devices Market.Available Customization
The analyst offers customization according to your specific needs. The following customization options are available for the report:- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The key players profiled in this Pregnancy Testing Devices market report include:- Abbott
- Roche
- BD
- Siemens Healthineers
- Beckman Coulter
- Acon Laboratories
- SD Biosensor
- Hologic
- AmniSure
- Quest Diagnostics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | January 2026 |
| Forecast Period | 2025 - 2031 |
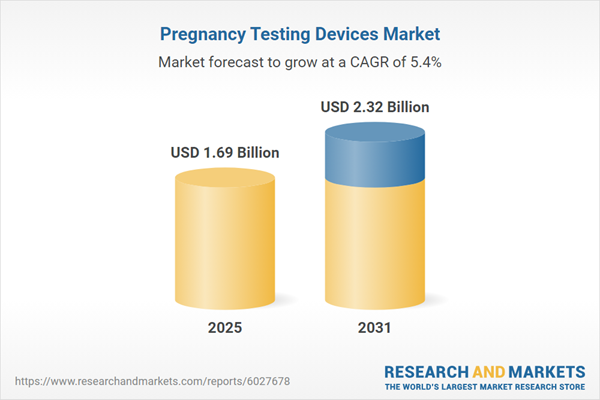
| Estimated Market Value ( USD | $ 1.69 Billion |
| Forecasted Market Value ( USD | $ 2.32 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |