Speak directly to the analyst to clarify any post sales queries you may have.
The umbilical catheter anchor market is evolving rapidly, shaped by ongoing innovation, shifting clinical requirements, and an increasingly complex regulatory landscape. For senior decision-makers seeking to optimize neonatal care protocols, understanding the fusion of advanced fixation technologies, material safety, and adaptable solutions is essential for strategic sourcing and product planning.
Market Snapshot: Umbilical Catheter Anchor Market Growth and Outlook
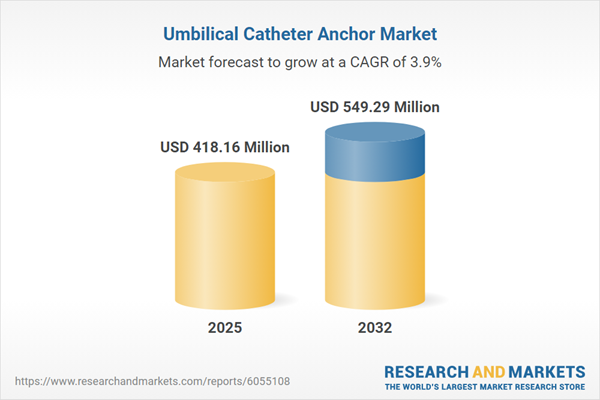
In 2024, the umbilical catheter anchor market was valued at USD 403.28 million, expanding to USD 418.16 million in 2025. This sector is set to maintain its upward trajectory at a compound annual growth rate (CAGR) of 3.93%, with projections indicating a market value of USD 549.29 million by 2032. Demand is driven by heightened safety expectations in neonatal care, advancing technological solutions, and evolving regulatory frameworks that support biocompatible, reliable, and sustainable anchoring systems.
Scope & Segmentation: Coverage Across Materials, Applications, and Geographies
This report delivers actionable insights on the umbilical catheter anchor market through robust analysis and market segmentation, including:
- Material: Hydrocolloid compounds, latex-free medical-grade polymers, polyurethane, silicone, and soft foam—each serving distinct needs for biocompatibility, flexibility, and patient comfort.
- Lumen Type: Double lumen, single lumen, and triple lumen configurations that enable customized administration of fluids and medications.
- Catheter Supported: Umbilical arterial catheters (UAC) and umbilical venous catheters (UVC), each tailored for specific neonatal procedures.
- End User: Ambulatory surgical centers, homecare settings, and hospitals—including neonatal intensive care units and pediatric departments—reflecting diverse use environments.
- Application: Drug administration, fluid therapies, and nutritional support underpinning neonatal care protocols.
- Distribution Channel: Offline and online avenues, mirroring established procurement methods and emerging rapid-delivery solutions.
- Geographic Regions: Americas (North America, Latin America), Europe, Middle East & Africa (including major European and Middle Eastern countries), Asia-Pacific (China, India, Japan, and other key economies).
- Leading Companies: ANGIPLAST PRIVATE LIMITED, Becton, Dickinson and Company, Boston Scientific Corporation, Cardinal Health, Inc., Coloplast Group, GPC Medical Ltd, Merit Medical Systems, Inc., Neotech Products LLC, Smiths Group plc, Vygon SA, McKesson Medical-Surgical Inc., and Medline Industries, LP.
Key Takeaways: Strategic Insights for Decision Makers
- Clinical demands are pushing manufacturers toward biocompatible, latex-free anchors that reduce allergic risks and improve patient outcomes.
- Smart anchoring technologies, such as integrated sensors and adaptive adhesives, support more precise catheter monitoring and can lower the frequency of clinical interventions.
- Segment-specific solutions—such as modular anchors for varying lumen counts—allow seamless care transitions and inventory standardization for healthcare providers.
- Sustainability is influencing both product design and procurement, with eco-friendly adhesives and recyclable packaging gaining traction among institutional buyers with environmental priorities.
- Digital integration, including app-based monitoring and clinician training platforms, not only enhances operational efficiency but also improves adherence to best practices in neonatal care.
- Partnerships with regional suppliers and contract manufacturers are crucial for responsive supply chains and accelerated regulatory compliance.
Tariff Impact: Navigating 2025's Material Cost Pressures
The introduction of 2025 tariffs on key medical materials has prompted organizations to rethink procurement and manufacturing strategies. Many device makers are now sourcing alternate raw materials and expanding local production to stabilize costs and protect supply continuity. In turn, procurement teams are focusing on bundled purchases and data-supported cost-effectiveness to navigate new budget constraints. These adaptations help safeguard patient safety and operational quality amid shifting financial pressures.
Methodology & Data Sources
The research combines primary interviews with neonatal care experts and procurement leaders, complemented by secondary reviews of regulatory filings, peer-reviewed studies, and technology adoption literature. Quantitative analysis of supply chain data, tariff impacts, and trade flows further supports the findings. Multi-stage workshops with industry stakeholders validate the relevance and accuracy of market insights.
Why This Report Matters
- Enables targeted product development and sourcing decisions aligned with the latest clinical and safety trends in neonatal care.
- Provides rigorous segmentation and regional analysis, supporting tailored go-to-market and procurement strategies for major stakeholders.
- Offers actionable intelligence for navigating regulatory shifts, technology adoption, and sustainability mandates in a highly specialized medical sector.
Conclusion
This report equips senior decision-makers with the insights needed to lead in the evolving umbilical catheter anchor market. By integrating clinical requirements, supply chain resilience, and technological innovation, organizations can enhance neonatal care delivery and competitive positioning.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Umbilical Catheter Anchor Market report include:- ANGIPLAST PRIVATE LIMITED
- Becton, Dickinson and Company
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Coloplast Group
- GPC Medical Ltd
- Merit Medical Systems, Inc.
- Neotech Products LLC
- Smiths Group plc
- Vygon SA
- McKesson Medical-Surgical Inc.
- Medline Industries, LP
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 183 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 418.16 Million |
| Forecasted Market Value ( USD | $ 549.29 Million |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |