Speak directly to the analyst to clarify any post sales queries you may have.
Personalized cell and gene therapies are ushering in a new era of precision medicine, transforming conventional approaches to disease management with targeted, patient-specific solutions. Senior decision-makers in the life sciences sector now face both immense opportunities and considerable complexity as these advanced modalities become increasingly central to the healthcare landscape.
Market Snapshot: Personalized Cell & Gene Therapies Market
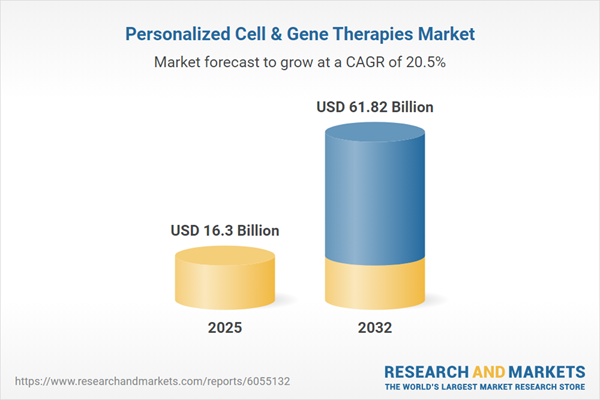
The Personalized Cell & Gene Therapies Market grew from USD 13.88 billion in 2024 to USD 16.30 billion in 2025 and is projected to continue expanding at a robust CAGR of 20.52%, reaching USD 61.82 billion by 2032.
This market trajectory reflects a rising demand for precise, adaptable therapies across diseases with significant unmet needs. Growth is being propelled by technological innovation, increased investment, and broadened clinical applications, while stakeholders navigate a complex regulatory and economic environment.Scope & Segmentation of the Personalized Cell & Gene Therapies Market
This report offers comprehensive coverage across the market, examining crucial drivers and segmented growth factors:
- Therapy Type: Cell Therapy, Gene Therapy
- Patient Type: Adult, Geriatric, Pediatric
- Therapeutic Area: Cardiology, Hematology, Immunology, Metabolic Diseases, Neurology, Oncology, Rare Diseases
- End User: Biopharmaceutical Companies, Hospitals & Clinics, Research Institutions
- Regional Coverage: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Key Technology Platforms: Genome editing (CRISPR, base editing), vector engineering, automated bioprocessing, closed system manufacturing, digital patient engagement
- Company Analysis: Adaptimmune Limited, Allogene Therapeutics, Amgen Inc., Arcellx, Inc., Beam Therapeutics Inc., BioCell Innovations, bluebird bio, Inc., Bristol-Myers Squibb Company, Caribou Biosciences, Inc., Celyad Oncology SA, CRISPR Therapeutics AG, Editas Medicine, Inc., Fate Therapeutics, Inc., Genentech, Inc., Gilead Sciences, Inc., Intellia Therapeutics, Inc., Lonza Group Ltd., Miltenyi Biomedicine GmbH, Novartis AG, Sana Biotechnology, Inc., Sangamo Therapeutics, Inc., Sarepta Therapeutics, Inc.
Key Takeaways for Decision Makers
- Technological breakthroughs in genome editing and cell manufacturing are shifting therapeutic development toward more individualized therapies.
- Collaborative ecosystems involving public-private partnerships and academic-industry alliances are crucial for advancing clinical programs and strengthening manufacturing capabilities.
- Segmentation by therapy modality, patient demographics, and therapeutic area shows that oncology leads commercialization, while novel approaches are emerging in neurology, immunology, and rare disease segments.
- Regional growth is strongest where regulatory innovation, investment incentives, and infrastructure development intersect, with Asia-Pacific and the Americas emerging as key hubs.
- Companies with vertically integrated platforms and early clinical collaborations are well-positioned to drive faster trial activation and broader patient access.
Tariff Impact on the Personalized Cell & Gene Therapies Market
Recent U.S. tariff adjustments for 2025 present both challenges and opportunities. Increased duties on critical reagents and equipment are pressuring margins and heightening the urgency for supply chain adaptation. Organizations are responding by nearshoring manufacturing, qualifying alternate suppliers, and investing in domestic production. This market shift supports investment in local biotech infrastructure and may yield operational benefits in the long term, although supply chain resilience remains a point of industry focus.
Methodology & Data Sources
This report's findings are based on a rigorous methodology, combining reviews of scientific literature, clinical trial databases, and regulatory documents with in-depth interviews of industry leaders. Quantitative metrics are cross-validated against independent data sources to ensure accuracy and robustness, with expert peer review integrated throughout.
Why This Report Matters
- Strategic insights empower leaders to adapt to evolving regulations, investment patterns, and competitive dynamics in personalized medicine.
- Actionable recommendations support smarter capital allocation, partnership decisions, and supply chain strategies, directly informing product development and commercialization plans.
- Nuanced segmentation and regional analysis reveal where to focus resources for maximum impact in a rapidly diversifying ecosystem.
Conclusion
The landscape of personalized cell and gene therapies is evolving rapidly, characterized by technological progress and increasing market complexity. Organizations that align innovation strategies with operational excellence and regional partner networks will be best positioned to capture growth and advance patient outcomes.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Personalized Cell & Gene Therapies market report include:- Adaptimmune Limited
- Allogene Therapeutics
- Amgen Inc.
- Arcellx, Inc.
- Beam Therapeutics Inc.
- BioCell Innovations
- bluebird bio, Inc.
- Bristol-Myers Squibb Company
- Caribou Biosciences, Inc.
- Celyad Oncology SA
- CRISPR Therapeutics AG
- Editas Medicine, Inc.
- Fate Therapeutics, Inc.
- Genentech, Inc.
- Gilead Sciences, Inc.
- Intellia Therapeutics, Inc.
- Lonza Group Ltd.
- Miltenyi Biomedicine GmbH
- Novartis AG
- Sana Biotechnology, Inc.
- Sangamo Therapeutics, Inc.
- Sarepta Therapeutics, Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 16.3 Billion |
| Forecasted Market Value ( USD | $ 61.82 Billion |
| Compound Annual Growth Rate | 20.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 23 |