Speak directly to the analyst to clarify any post sales queries you may have.
The personalized nanotechnology for cell and gene therapies market is rapidly evolving, driven by a convergence of precision nanotechnology, advanced cellular engineering, and collaborative innovation. Senior decision-makers navigating this space will find strategic value in understanding the diverse trends, regulatory responses, and regional dynamics shaping future opportunities.
Market Snapshot: Personalized Nanotechnology for Cell & Gene Therapies
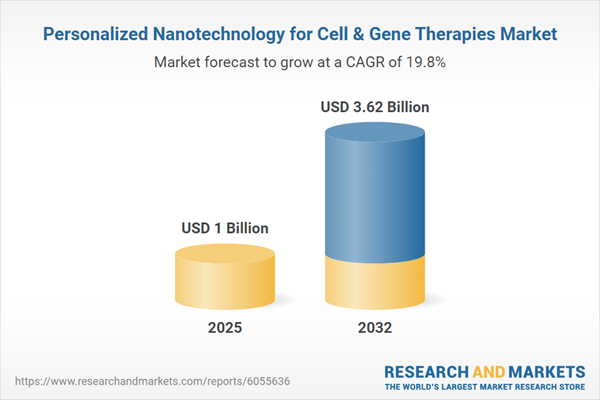
The Personalized Nanotechnology for Cell & Gene Therapies Market grew from USD 855.08 million in 2024 to USD 1.00 billion in 2025. It is expected to maintain a strong CAGR of 19.78%, reaching USD 3.62 billion by 2032. This remarkable growth reflects expanding integration of nanoscale delivery systems with cutting-edge gene editing and cell therapies, as innovators redefine manufacturing, clinical, and regulatory processes for next-generation therapeutic solutions.
Scope & Segmentation
This report delivers a thorough analysis and forecast across multiple dimensions, offering senior leaders actionable insights on pressing market segments and their strategic impact.
- Therapy Types: Immune cell therapy, induced pluripotent stem cells, mesenchymal stem cells, stem cell therapy, CRISPR technology, delivery mechanisms, gene modification methods, non-viral vectors.
- Nanotechnology Types: Nanocarrier design, nanodevices, nanoencapsulation, nanomaterials, nanomedicine, nanostructure imaging.
- Applications: Cancer therapies, cardiovascular diseases, inherited genetic disorders, neurological disorders.
- End Users: Diagnostic centers, healthcare facilities, pharmaceutical companies, research institutions.
- Regions Covered: Americas (North America: United States, Canada, Mexico; Latin America: Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (Europe: United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland; Middle East: United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel; Africa: South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Companies Reviewed: AbbVie Inc., Amgen Inc., Arcturus Therapeutics Inc., AstraZeneca PLC, BASF Pharma Solutions, BlueWillow Biologics Inc., Bristol-Myers Squibb Company, Camurus AB, Celgene Inc., Cristal Therapeutics, Cytimmune Sciences, Eisai Co. Ltd., EyePoint Pharmaceuticals, Gilead Sciences Inc., GlaxoSmithKline PLC, Hoffmann-La Roche Ltd, Ipsen Pharma, Johnson & Johnson, Merck KGaA, Nanobiotix SA, Novartis AG, Pfizer Inc., Spark Therapeutics Inc., Teva Pharmaceutical Industries Limited, Thermo Fisher Scientific Inc.
Key Takeaways for Decision-Makers
- Programmable nanocarriers and new molecular engineering techniques are enabling deeper personalization of cell and gene therapies across diverse patient populations.
- Immune cell engineering, supported by advanced nanomaterials, is driving innovation in targeted treatments and reducing adverse immune responses.
- Collaborative models, including academic-industry alliances and public-private partnerships, are accelerating research, scale-up, and commercial pipeline development.
- Modular manufacturing and digital health integration are shortening development cycles and enhancing quality control, supporting seamless technology transfer from laboratory to clinic.
- Oncology leads application areas, but cardiovascular, neurological, and inherited genetic disorder segments are expanding as nanotechnology-based precision delivery platforms mature.
- Regulatory frameworks are adapting, with agencies implementing adaptive pathways to balance patient safety with rapid market access for transformative therapies.
Tariff Impact on Nanotechnology Supply Chains
Recent United States tariffs on specialized nanotechnology components are reshaping global supply chains. Many organizations are responding by investing in domestic manufacturing and forming regional partnerships to counteract cost pressures and mitigate risks tied to raw material sourcing. These shifts are fostering greater resilience and new strategic alliances across value chains, ultimately supporting uninterrupted innovation and patient access.
Methodology & Data Sources
The report combines primary interviews with key opinion leaders, a broad literature review including scientific, regulatory, and patent sources, and advanced analytics leveraging trade and clinical data. Expert validation ensures all recommendations are grounded, balanced, and relevant to executive priorities.
Why This Report Matters
- Equips decision-makers with actionable intelligence covering segment trends, emerging technologies, and evolving regulatory environments.
- Supports strategic planning by elucidating regional variations and key competitive moves, helping prioritize investments and partnerships.
- Drives proactive risk management by highlighting tariff-induced supply chain disruptions and mitigation strategies.
Conclusion
Personalized nanotechnology is reshaping cell and gene therapy innovation globally. Informed, cross-functional strategies will empower stakeholders to address complexities, capitalize on market opportunities, and deliver patient-centric solutions at scale.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Personalized Nanotechnology for Cell & Gene Therapies market report include:- AbbVie Inc.
- Amgen Inc.
- Arcturus Therapeutics, Inc.
- AstraZeneca PLC
- BASF Pharma Solutions
- BlueWillow Biologics Inc.
- Bristol-Myers Squibb Company
- Camurus AB
- Celgene, Inc.
- Cristal Therapeutics
- Cytimmune Sciences
- Eisai Co., Ltd.
- EyePoint Pharmaceuticals
- Gilead Sciences Inc.
- GlaxoSmithKline PLC
- Hoffmann-La Roche Ltd
- Ipsen Pharma
- Johnson & Johnson
- Merck KGaA
- Nanobiotix SA
- Novartis AG
- Pfizer Inc.
- Spark Therapeutics, Inc.
- Teva Pharmaceutical Industries Limited
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 188 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1 Billion |
| Forecasted Market Value ( USD | $ 3.62 Billion |
| Compound Annual Growth Rate | 19.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |