Speak directly to the analyst to clarify any post sales queries you may have.
Sterile PVA eye spears are advancing the standard of ophthalmic consumables by delivering reliable, safe, and efficient tools for both routine and surgical vision care. As the primary keyword, the sterile PVA eye spear market is positioned as a core driver of greater procedural consistency and patient safety in clinical practice.
Market Snapshot: Sterile PVA Eye Spear Market Growth Outlook
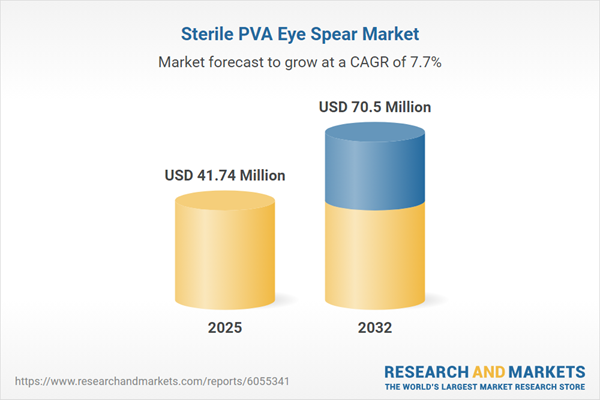
The sterile PVA eye spear market grew from USD 38.92 million in 2024 to USD 41.74 million in 2025. With a compound annual growth rate (CAGR) of 7.71%, the sector is projected to achieve USD 70.50 million by 2032. This pace reflects sustained investment in ophthalmic consumables as stakeholders in hospitals, surgical centers, and specialty clinics prioritize improved infection control, patient comfort, and supply reliability.
Scope & Segmentation
The scope of this report encompasses the full value chain of sterile PVA eye spears, from manufacturing innovation to global delivery models. Each market segment is detailed to support targeted strategy development across products, materials, sterilization processes, applications, and geographies. Key segmentation points include:
- Product: Micro-applicators, PVA eye spears, sponge eye spears.
- Material: Natural PVA, synthetic PVA.
- Sterilization Method: E-beam sterilized, gamma sterilized.
- Application: Non-surgical procedures (eye cleaning, postoperative care), surgical procedures (cataract surgery, ophthalmic surgeries, refractive surgery).
- End-User: Ambulatory surgical centers, homecare settings, hospitals, specialty clinics.
- Distribution Channel: Offline, online.
- Region: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Key Companies: Accutome Inc., Aegis Lifesciences, Bausch + Lomb Incorporated, Beaver-Visitec International Ltd., Carwild Corporation, DeRoyal Industries Inc., Devine Meditech, Huizhou Foryou Medical Devices Co. Ltd., Jiande Kanghua Medical Devices Co. Ltd., Labtician Ophthalmics Inc., Madhu Instruments Pvt. Ltd., Oasis Medical Inc., OPHTECHNICS UNLIMITED, SDP Inc., SURGITECH INNOVATION.
Key Takeaways for Senior Decision-Makers
- Material selection—between natural and synthetic PVA—remains a critical choice, balancing softness and tensile strength with procedure requirements.
- Sterile PVA eye spears support both advanced surgery and decentralized care, aligning with the shift toward homecare and ambulatory settings for ophthalmic treatment.
- Technological advancements in sterilization and tamper-evident packaging are supporting regulatory compliance and traceability across international distribution channels.
- Regional strategies must account for varying regulatory standards and the increasing demand for local manufacturing partnerships in emerging economies.
- Digital supply chain integrations and online distribution channels are becoming central to procurement for health institutions, providing real-time visibility and inventory agility.
Tariff Impact and Strategic Response
The introduction of new United States tariffs in 2025 created considerable complexity within supply chains. Import duties affected raw materials and finished sterile PVA eye spears, leading to recalibrations in supplier and manufacturing strategies. Stakeholders have responded through joint ventures, reshoring initiatives, and negotiation of revised logistics agreements, all of which promote supply continuity and cost control. These adaptive measures have reinforced industry resilience amid evolving trade environments.
Methodology & Data Sources
This report utilizes a rigorous multi-source research framework. The approach combines qualitative expert interviews, secondary data from regulatory filings and trade reports, and on-site supply chain assessments. Findings were cross-validated and subject to peer review, with complete methodological transparency to ensure data integrity throughout the analysis.
Why This Report Matters
- Enables senior leaders to benchmark product and technology differentiation across all major sterile PVA eye spear categories and end-markets.
- Guides investment, procurement, and regulatory strategies with actionable insights on evolving market drivers and competitive dynamics.
- Supports long-range planning by offering comprehensive segmentation analysis, including critical tariff and regional factors impacting the supply landscape.
Conclusion
This analysis affirms that sterile PVA eye spears remain vital to advancing both clinical efficiency and patient-centered care in ophthalmology. Stakeholders who leverage these market insights can strengthen strategic positioning and sustain growth in an increasingly dynamic global environment.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Sterile PVA Eye Spear market report include:- Accutome Inc.
- Aegis Lifesciences
- Bausch + Lomb Incorporated
- Beaver-Visitec International, Ltd.
- Carwild Corporation
- DeRoyal Industries, Inc.
- Devine Meditech
- Huizhou Foryou Medical Devices Co., Ltd.
- Jiande Kanghua Medical Devices, Co., Ltd.
- Labtician Ophthalmics, Inc.
- Madhu Instruments Pvt. Ltd.
- Oasis Medical Inc.
- OPHTECHNICS UNLIMITED
- SDP Inc.
- SURGITECH INNOVATION
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 193 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 41.74 Million |
| Forecasted Market Value ( USD | $ 70.5 Million |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 16 |