Speak directly to the analyst to clarify any post sales queries you may have.
The CHAPLE Disease market is undergoing rapid transformation as next-generation therapeutics, advanced diagnostics, and collaborative care models converge. Senior healthcare and biopharmaceutical decision-makers are seeking actionable insights to navigate this evolving landscape and drive improved outcomes, operational efficiency, and targeted investment.
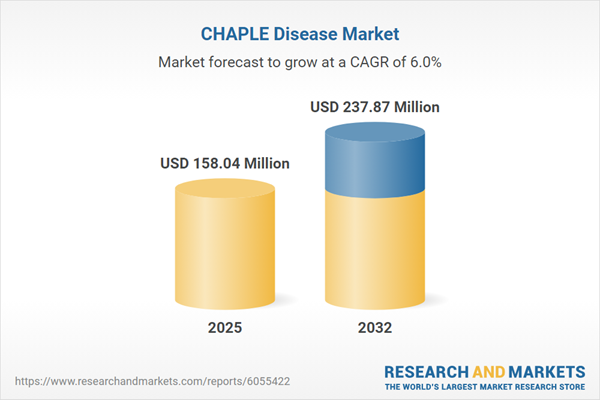
Market Snapshot: CHAPLE Disease Market Size and Growth
The CHAPLE Disease market expanded from USD 149.56 million in 2024 to USD 158.04 million in 2025 and is expected to continue at a CAGR of 5.97%, attaining USD 237.87 million by 2032. This sustained growth reflects rising awareness, increased adoption of precision therapies, and strengthening diagnostic capabilities worldwide. Market dynamics are influenced by regulatory developments, evolving treatment paradigms, and ongoing stakeholder collaborations across the Americas, EMEA, and Asia-Pacific.
Scope & Segmentation
This comprehensive report delivers an in-depth analysis spanning emerging care models, clinical advances, and regional strategy. The research encompasses the full spectrum of disease management, from diagnosis through treatment, distribution, and end-user settings. Key segments include:
- Type: Type 1 Chaple Syndrome, Type 2 Chaple Syndrome, Type 3 Chaple Syndrome, Type 4 Chaple Syndrome, Type 5 Chaple Syndrome
- Offering: Clinical Evaluation, Genetic Testing, Eculizumab, Ravulizumab, Veopoz
- Route of Administration: Intravenous, Oral, Subcutaneous
- Dosage Form: Lyophilized, Tablet
- End User: Clinics, Home Care, Hospitals, Research Institutes
- Distribution Channel: Offline, Online
Regional breakdown details all key geographies, including:
- Americas: United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru
- Europe, Middle East & Africa: United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya
- Asia-Pacific: China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan
The report examines leading companies: Akari Therapeutics, Alexion Pharmaceuticals (AstraZeneca), Alnylam Pharmaceuticals, Apellis Pharmaceuticals, CinnaGen, and Regeneron Pharmaceuticals, Inc.
Key Takeaways for CHAPLE Disease Market Stakeholders
- Precision medicine and rapid genetic diagnostics are accelerating initial CHAPLE Disease identification, minimizing delayed interventions for patients and improving clinical outcomes.
- Next-generation complement inhibitors with extended dosing are transforming disease control, enabling greater patient adherence and streamlined care delivery.
- Patient-centric models such as home-based infusion and telemedicine are enhancing access, particularly in markets facing logistical or infrastructural constraints.
- Strategic public-private collaborations foster innovation and data-driven care, while helping overcome reimbursement, regulatory, and access barriers across diverse healthcare settings.
- Tailored segmentation analysis empowers companies to align new products with the needs of specific phenotypes, administration preferences, and evolving distribution infrastructures.
Tariff Impact: Implications of 2025 U.S. Biologic Import Policy
The 2025 United States tariffs on biologic imports are poised to influence the economics of CHAPLE Disease therapies. Import duties may prompt recalibration of supply chain strategies—shifting purchasing, sourcing, and reimbursement models. Providers may explore new supplier relationships or localized production to mitigate increased costs, while patient assistance and financial support initiatives are expected to intensify to sustain access. Industry adaptability in manufacturing, advocacy, and procurement will be critical to ensuring therapeutic continuity in this changing environment.
Methodology & Data Sources
This analysis integrates expert interviews, systematic literature review, and comprehensive real-world evidence from registries and hospital databases. Data are synthesized using advanced competitive intelligence tools and validated through robust triangulation, ensuring strategic reliability for institutional stakeholders.
Why This Report Matters
- Enables data-driven investment and R&D prioritization by illuminating clinical, commercial, and regulatory trends relevant to CHAPLE Disease market evolution.
- Equips leaders to respond proactively to cost, reimbursement, and logistical uncertainties, safeguarding patient access and supply resilience.
- Supports targeted product development and stakeholder engagement strategies in highly segmented and dynamic regional environments.
Conclusion
The CHAPLE Disease market demands cross-functional coordination, adaptive supply chain management, and ongoing innovation. By leveraging this report, decision-makers gain a competitive edge in optimizing strategies that support both near- and long-term market opportunities.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this CHAPLE Disease Market report include:- Akari Therapeutics
- Alexion Pharmaceuticals, Inc by AstraZeneca plc
- Alnylam Pharmaceuticals, Inc.
- Apellis Pharmaceuticals, Inc.
- CinnaGen Co.
- Regeneron Pharmaceuticals, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 158.04 Million |
| Forecasted Market Value ( USD | $ 237.87 Million |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 7 |