Speak directly to the analyst to clarify any post sales queries you may have.
The global fibrotic diseases treatment market is evolving rapidly, driven by advances in targeted therapies, diagnostics, and patient monitoring solutions. Senior decision-makers require clear, actionable insights to optimize investments and strategies within this dynamic environment.
Market Snapshot: Fibrotic Diseases Treatment Overview
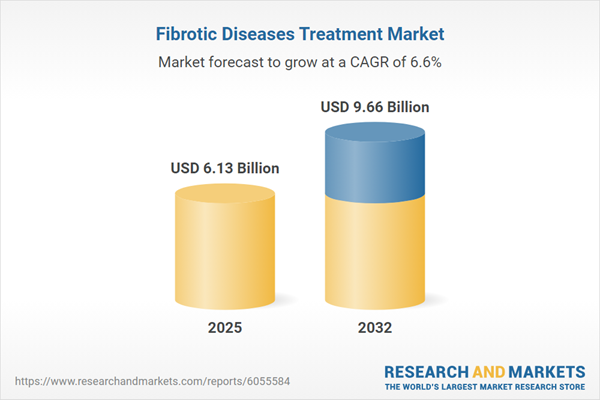
The fibrotic diseases treatment market grew from USD 5.78 billion in 2024 to USD 6.13 billion in 2025 and is projected to maintain a CAGR of 6.63%, reaching USD 9.66 billion by 2032. This sustained expansion reflects significant unmet needs across chronic cardiac, pulmonary, hepatic, and renal conditions. Demand is reinforced by aging populations, increasing metabolic disorders, and environmental risk factors, all of which highlight the urgency for innovative and effective therapies in fibrotic disease management.
Scope & Segmentation
- Disease Types: Cardiac fibrosis, liver fibrosis, pulmonary fibrosis, and renal fibrosis―each involving distinct pathophysiological mechanisms and therapeutic requirements.
- Treatment Types: Medication, organ transplantation, and oxygen therapy, supporting the full care continuum from early intervention to management of late-stage complications.
- Administration Methods: Inhalation, injection, intravenous, oral, and topical routes, facilitating both systemic and localized delivery tailored to disease site and severity.
- Treatment Stages: Advanced-stage fibrosis, early-stage fibrosis, end-stage organ failure, and pre-fibrosis (preventative treatment) to address the progression continuum and optimize outcomes.
- End Users: Academic and research institutes, hospitals, and specialty clinics, reflecting the collaborative ecosystem essential for innovation and commercial success.
- Geographic Coverage: Americas (including United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland), Middle East (United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel), Africa (South Africa, Nigeria, Egypt, Kenya), and Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan).
- Key Players: AbbVie Inc., Amgen Inc., Baxter International Inc., Biogen Inc., Boehringer Ingelheim International GmbH, Bristol-Myers Squibb Company, Eli Lilly and Company, Ferring Pharmaceuticals, Gilead Sciences, GlaxoSmithKline, Ipsen Pharma, Kyorin Pharmaceutical, Mallinckrodt, Merck & Co., Mitsubishi Tanabe Pharma, Novartis, Pfizer, Regeneron, Sanofi, Santen Pharmaceutical, Takeda, Teva, UCB S.A.
Key Takeaways for Strategic Decision-Makers
- Precision therapies targeting fibrogenic pathways are rapidly replacing traditional nonspecific options, enabling more personalized and effective management.
- Innovations in gene editing and RNA interference show promise in altering disease progression, with early clinical data supporting potential clinical impact.
- Integrated diagnostic solutions, including biomarker-driven companion diagnostics and digital health tools, enhance patient stratification and monitoring capabilities.
- Collaborative frameworks between pharmaceutical, biotech, and technology firms are expediting therapeutic development and improving commercial outcomes.
- The diversity of administration modalities allows for customized regimens to enhance adherence and minimize adverse effects in chronic management settings.
- Regional adaptation remains critical, as regulatory, reimbursement, and healthcare infrastructure differences shape adoption rates and market access strategies worldwide.
Tariff Impact on Supply Chain and Market Access
Newly implemented United States tariffs have driven manufacturers to reconsider their procurement and manufacturing strategies, with a growing focus on strengthening domestic supply capability and minimizing potential disruptions. These changes are prompting firms to invest in advanced manufacturing technologies and diversify supply networks, while also influencing negotiation strategies with contract manufacturing partners and research collaborators. As a result, supply chain risk management has become an executive priority, underlining the need for operational resilience and adaptability.
Methodology & Data Sources
This report combines primary interviews with clinicians, industry experts, and executive leaders, as well as secondary data from peer-reviewed publications, regulatory sources, trial registries, patent filings, and company reports. Advanced analytics were applied to assess therapy adoption, clinical trends, and pricing dynamics, ensuring robust, triangulated insights for critical decision-making.
Why This Report Matters
- Equips leaders to anticipate paradigm shifts, prioritize R&D, and optimize resource deployment for maximum competitive advantage.
- Facilitates informed partnership selection by providing detailed segmentation and regional perspectives tailored to varied regulatory and market environments.
- Delivers actionable guidance on technology adoption, supply chain management, and commercialization pathways specific to fibrotic disease therapeutics.
Conclusion
Senior executives and strategic planners can rely on this comprehensive analysis to navigate the evolving fibrotic diseases treatment market. Through data-driven insights and targeted recommendations, organizations are positioned to drive innovation, form impactful partnerships, and enhance patient outcomes in this complex therapeutic arena.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Fibrotic Diseases Treatment market report include:- AbbVie Inc.
- Amgen Inc.
- Baxter International Inc.
- Biogen Inc.
- Boehringer Ingelheim International GmbH
- Bristol-Myers Squibb Company
- Eli Lilly and Company
- Ferring Pharmaceuticals Private Limited
- Gilead Sciences, Inc.
- GlaxoSmithKline plc
- Ipsen Pharma
- Kyorin Pharmaceutical Co., Ltd.
- Mallinckrodt
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novartis International AG
- Pfizer Inc.
- Regeneron Pharmaceuticals, Inc.
- Sanofi S.A.
- Santen Pharmaceutical Co., Ltd.
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceutical Industries Ltd.
- UCB S.A.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 189 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 6.13 Billion |
| Forecasted Market Value ( USD | $ 9.66 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |