Speak directly to the analyst to clarify any post sales queries you may have.
Embarking on a Transformative Exploration of Dental Soft-Tissue Regeneration Unveiling Key Technological Breakthroughs Market Drivers Emerging Clinical Priorities Strategic Innovations and Industrywide Growth Imperatives
Over the past decade, the field of dental soft-tissue regeneration has evolved from a niche clinical practice to a cornerstone of comprehensive oral rehabilitation strategies. Initially driven by the need to mitigate the limitations of traditional autologous grafting, innovation in biomaterials science has introduced a spectrum of collagen-based membranes, synthetic scaffolds, and advanced gels that facilitate predictable tissue integration and accelerated healing trajectories. Moreover, the synergy between regenerative engineering and growth factor delivery systems has ushered in a new era of personalized therapy, enabling clinicians to tailor interventions to unique patient profiles and defect geometries.In addition, the convergence of digital imaging, three-dimensional printing, and minimally invasive surgical techniques has redefined procedural workflows, enhancing precision while reducing patient morbidity. As a result, practitioners are now leveraging custom-designed scaffolds and biologically active membranes that align seamlessly with anatomical contours. Furthermore, the integration of patient-centric care models emphasizes not only clinical outcomes but also aesthetic and functional restoration as key success metrics. Consequently, strategic innovations in tissue engineering are positioned to address unmet needs, reinforcing the industry's commitment to elevating standards of oral health. This executive summary synthesizes these dynamics and frames the insights that will guide stakeholders through an increasingly complex landscape of clinical applications, regulatory considerations, and collaborative growth imperatives.
Reimagining the Landscape of Dental Soft-Tissue Regeneration Through Converging Technological Disruptions Patient-Centric Care Models Innovative Therapeutic Modalities and Regulatory Evolution
Recent years have witnessed a profound reimagining of the dental soft-tissue regeneration landscape, propelled by a wave of technological disruptions that span from additive manufacturing platforms to bioactive nanocomposite materials. High-resolution imaging integrated with computer-aided design workflows has enabled the fabrication of patient-specific membranes and scaffolds, while advances in cell culture and bioprinting techniques have expanded the repertoire of viable tissue constructs. Additionally, the incorporation of growth factors, peptides, and gene delivery vectors into these scaffolds has activated intrinsic healing pathways, thereby reducing reliance on donor-derived grafts and minimizing immunogenic risk.Furthermore, a shift toward patient-centric care models has underscored the importance of minimally invasive protocols, rapid postoperative recovery, and long-term functional outcomes. In parallel, regulatory evolution has streamlined pathways for regenerative products through designations that foster accelerated review and clinical adoption. Stakeholders are responding by forging collaborative ecosystems that unite academic research, industry partners, and clinical networks to validate emerging modalities. As a result, the industry is entering an era characterized by multidisciplinary innovation, enhanced procedural efficiency, and a redefinition of success metrics that extend beyond tissue closure to encompass patient satisfaction and quality-of-life improvements.
These emergent shifts highlight the critical role of cross-disciplinary collaboration in translating laboratory breakthroughs into standardized clinical protocols and improved patient experiences
Assessing the Comprehensive Impact of United States Tariffs on Dental Soft-Tissue Regeneration Supply Chains Manufacturing Costs Strategic Responses and Market Dynamics in 2025
The imposition of new United States tariffs in 2025 on key raw materials and components has introduced fresh complexities to the supply chains underpinning dental soft-tissue regeneration. Imported collagen matrices, specialized polymers, and interactive membranes are now subject to elevated duties, resulting in incremental cost pressures for manufacturers and distributors. These levies coincide with broader shifts in global trade policies, compelling stakeholders to reassess sourcing strategies and optimize inventory management to maintain competitive pricing for regenerative products.As a direct consequence, industry players are accelerating efforts to diversify their material portfolios and reduce reliance on a limited set of suppliers. Some organizations are advancing domestic production capabilities, pursuing vertical integration to capture value and mitigate exposure to tariff fluctuations. Others are exploring alternative biomaterial platforms and synthetic analogues that can deliver comparable biological performance at a more stable cost base. In conjunction with these approaches, strategic alliances are emerging to share manufacturing resources and distribute the financial burden of regulatory compliance. Therefore, the interplay of tariff-driven cost adjustments and supply network realignment is poised to redefine competitive dynamics and influence the allocation of research and development investments across the dental regeneration sector.
Ultimately, agility in supply chain design and proactive policy navigation will be critical to sustaining growth trajectories amidst evolving trade landscapes
Unveiling Critical Market Segmentation Insights in Dental Soft-Tissue Regeneration Across Product Innovation Techniques Clinical Applications Strategic Differentiation and End-Use Environments
The product landscape in dental soft-tissue regeneration encompasses a progression from collagen-based membranes leveraging natural extracellular matrix properties to sophisticated gels and biomaterials engineered for controlled bioactive delivery. Scaffold technologies have been refined to offer customizable architectures that support cellular ingrowth and vascular integration, while soft tissue grafts now include synthetic biomaterials and comprehensive tissue engineering solutions designed to minimize donor site morbidity. This spectrum of innovation reflects a strategic imperative for differentiation among regenerative platforms.In terms of procedural diversity, techniques span autogenous graft harvesting, where patients' own tissues are repurposed, as well as allogeneic approaches utilizing donor-derived materials to reduce surgical invasiveness. Alloplastic and xenogeneic options further broaden therapeutic choices, with guided tissue regeneration protocols providing precise spatial guidance for optimal tissue ingrowth. Clinical applications are equally multifaceted, addressing periodontal regeneration to rebuild supporting structures around dentition, reconstructive surgery that targets maxillofacial defects and soft tissue augmentation for esthetic enhancement, as well as wound healing strategies tailored to both acute injuries and chronic soft tissue deficiencies. These interventions are deployed across diverse end-use environments, from specialized dental clinics performing routine regenerative procedures to hospital settings equipped for complex surgical workflows, with research institutions serving as key incubators for validating next-generation therapies and translating laboratory discoveries into clinical practice.
Decoding Regional Dynamics in Dental Soft-Tissue Regeneration With Focus on Americas Diverse EMEA Markets Emerging Asia-Pacific Growth Opportunities Clinical and Commercial Realities
In the Americas, the dental soft-tissue regeneration sector benefits from well-established clinical infrastructures and a high degree of reimbursement support for regenerative therapies. Leading academic centers and private practices are early adopters of innovative biomaterials and guided tissue regeneration protocols, fostering a competitive environment that incentivizes continuous product development. Meanwhile, the Europe, Middle East & Africa region presents a heterogeneous mix of regulatory landscapes, where centralized approval mechanisms coexist with country-specific reimbursement frameworks. This diversity creates both challenges and opportunities for companies seeking market entry, as localized clinical evidence and health economic assessments become critical determinants of adoption rates.Conversely, the Asia-Pacific region is characterized by rapidly expanding dental service networks and increasing investments in clinical training and research. Emerging economies are demonstrating particular enthusiasm for cost-effective regenerative solutions, driving demand for synthetic scaffolds and autologous graft alternatives that align with local healthcare budgets. Strategic partnerships between global manufacturers and regional distributors are facilitating technology transfer and capacity building, while urban centers in developed markets are advancing high-end restorative procedures supported by cutting-edge imaging and surgical platforms. As a result, Asia-Pacific is poised to influence global innovation agendas and shape future standards of care through a blend of affordability and technological prowess.
Profiling Key Industry Leaders and Emerging Innovators Driving Breakthroughs in Dental Soft-Tissue Regeneration Through Strategic Collaborations Intellectual Property Leadership and Clinical Pipeline Excellence
A cadre of industry leaders has emerged at the forefront of dental soft-tissue regeneration, distinguished by robust intellectual property portfolios and extensive clinical pipelines. Major players have leveraged proprietary collagen formulations, advanced polymer blends, and customized scaffold design software to establish defensible market positions. These organizations frequently engage in strategic collaborations with academic institutions and contract research organizations to expedite clinical validation and regulatory submissions. Their capacity to integrate advanced manufacturing processes with rigorous quality management systems underpins a competitive advantage in delivering reliable regenerative products at scale.Alongside established firms, a wave of emerging innovators is reshaping competitive dynamics by introducing next-generation biomaterials and cell-based constructs that promise enhanced biological performance. Collaborative consortia and joint ventures are becoming commonplace as smaller enterprises seek to align technological breakthroughs with the distribution networks and regulatory expertise of larger partners. Moreover, cross-industry alliances with digital dentistry and surgical robotics providers are creating integrated solutions that streamline procedural workflows. This trend underscores the importance of ecosystem-based strategies, where shared resources and complementary competencies accelerate time to market and foster sustainable innovation.
Actionable Strategic Recommendations for Industry Leaders to Accelerate Adoption of Advanced Dental Soft-Tissue Regeneration Technologies Enhance Market Access and Foster Sustainable Growth Trajectories
To capitalize on the evolving landscape of dental soft-tissue regeneration, industry leaders should prioritize investments in next-generation biomaterials that offer modularity and adaptability across diverse clinical scenarios. Establishing multidisciplinary teams that blend material science expertise with surgical know-how will accelerate innovation cycles and ensure that product development remains aligned with end-user requirements. In addition, forging partnerships with academic research centers and clinical key opinion leaders can facilitate early-stage validation and generate real-world evidence that supports favorable reimbursement outcomes.Moreover, organizations must reinforce supply chain resilience by diversifying sourcing channels and exploring regional manufacturing facilities in established and emerging markets. Engaging proactively with regulatory authorities to define clear pathways for combination products and biologic-device interfaces will mitigate approval risks. Embracing value-based care models that quantify patient-centric outcomes, such as functional recovery and quality-of-life improvements, will strengthen market access propositions. Finally, investing in clinician education programs to disseminate best practices and procedural updates will promote consistent adoption and optimize therapeutic success, thereby establishing a solid foundation for sustained growth.
Additionally, embracing digital health platforms for remote monitoring and outcome tracking can yield rich real-world evidence, informing iterative improvements and reinforcing patient engagement across the care continuum.
Elucidating the Research Methodology Underpinning Dental Soft-Tissue Regeneration Insights Featuring Robust Primary Interviews Rigorous Secondary Analysis Analytical Modeling and Comprehensive Data Validation Frameworks
The insights presented in this report are grounded in a rigorous research methodology that integrates primary and secondary data sources to provide an authoritative perspective on dental soft-tissue regeneration. Primary research involved in-depth interviews with leading clinicians, material scientists, and regulatory experts to capture experiential knowledge and anticipate future trends. These qualitative inputs were complemented by extensive secondary analysis of scientific literature, patent filings, clinical trial registries, and regulatory guidelines to construct a comprehensive evidence base.To ensure analytical rigor, data triangulation techniques were employed to reconcile divergent findings and validate thematic inferences. Specialized modeling tools facilitated segmentation analysis across product types, techniques, applications, and end-use environments, while sensitivity assessments tested the robustness of identified patterns. Peer review processes and iterative stakeholder consultations further refined the conclusions, ensuring that recommendations are both actionable and contextually relevant. Throughout this process, adherence to ethical research practices and transparency standards was maintained, reinforcing the credibility of the report's insights and supporting strategic decision-making for stakeholders across the dental regeneration ecosystem.
Quantitative benchmarking of product performance metrics was conducted to support comparative analysis, while qualitative thematic coding of expert interviews ensured the capture of nuanced practitioner perspectives. This multilayered approach establishes a robust foundation for strategic decision-making.
Concluding Perspectives on the Future of Dental Soft-Tissue Regeneration Emphasizing Innovation Trajectories Strategic Imperatives and the Pathway to Clinical Excellence and Market Leadership
In synthesizing the multifaceted developments in dental soft-tissue regeneration, it becomes evident that innovation is progressing along convergent trajectories that blend material science breakthroughs, procedural refinements, and patient-centric care paradigms. The maturation of scaffold technologies, coupled with the integration of biologically active agents, has opened avenues for more predictable and durable outcomes. Simultaneously, evolving regulatory frameworks and shifting trade policies underscore the necessity for agile operational strategies and robust cross-functional collaboration. As a result, stakeholders are presented with a window of opportunity to redefine treatment standards and deliver differentiated value across clinical, commercial, and research domains.Looking ahead, the focus will increasingly center on marrying technological sophistication with cost-effective delivery models, ensuring that advanced regenerative therapies are accessible to broader patient populations. Strategic alignment between innovators, clinicians, and payers will be essential in establishing clear value propositions and fostering meaningful adoption. Ultimately, the successful navigation of this dynamic landscape hinges on a commitment to continuous learning, proactive engagement with policy environments, and an unyielding dedication to improving oral health outcomes through scientific excellence. These concluding perspectives aim to guide decision-makers in charting a course toward clinical leadership and sustainable impact.
Market Segmentation & Coverage
This research report forecasts the revenues and analyzes trends in each of the following sub-segmentations:- Product Type
- Collagen-Based Membranes
- Gels and Biomaterials
- Scaffolds
- Soft Tissue Grafts
- Synthetic Biomaterials
- Tissue Engineering Solutions
- Technique
- Allogeneic Techniques
- Alloplastic Techniques
- Autogenous Techniques
- Guided Tissue Regeneration (GTR)
- Xenogeneic Techniques
- Application
- Periodontal Regeneration
- Reconstructive Surgery
- Maxillofacial Reconstruction
- Soft Tissue Augmentation
- Soft Tissue Wound Healing
- Acute Wound Healing
- Chronic Wound Healing
- End-Use
- Dental Clinics
- Hospitals
- Research Institutions
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- B. & B. Dental S.R.L
- Bego GmbH Co. KG
- Biomatlante SA
- Botiss Biomaterials GmbH
- DENTSPLY SIRONA Inc.
- Envista Holdings Corporation
- Geistlich Pharma AG
- Henry Schein, Inc
- Keystone Dental Group
- Maxigen Biotech, Inc.
- Meccellis Biotech
- Medical Consult Implants GmbH
- Neoss AG
- Orthogen LLC
- Osstem Implant Co., Ltd.
- Osteogenics Biomedical
- Regedent AG
- Regenity Biosciences
- Sinai Dental Group
- Straumann Holding AG
- Sunstar Suisse SA
- Tissue Regenix Ltd.
- Zimmer Biomet Holdings, Inc.
- Zimvie Inc.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Dental Soft-Tissue Regeneration market report include:- B. & B. Dental S.R.L
- Bego GmbH Co. KG
- Biomatlante SA
- Botiss Biomaterials GmbH
- DENTSPLY SIRONA Inc.
- Envista Holdings Corporation
- Geistlich Pharma AG
- Henry Schein, Inc
- Keystone Dental Group
- Maxigen Biotech, Inc.
- Meccellis Biotech
- Medical Consult Implants GmbH
- Neoss AG
- Orthogen LLC
- Osstem Implant Co., Ltd.
- Osteogenics Biomedical
- Regedent AG
- Regenity Biosciences
- Sinai Dental Group
- Straumann Holding AG
- Sunstar Suisse SA
- Tissue Regenix Ltd.
- Zimmer Biomet Holdings, Inc.
- Zimvie Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
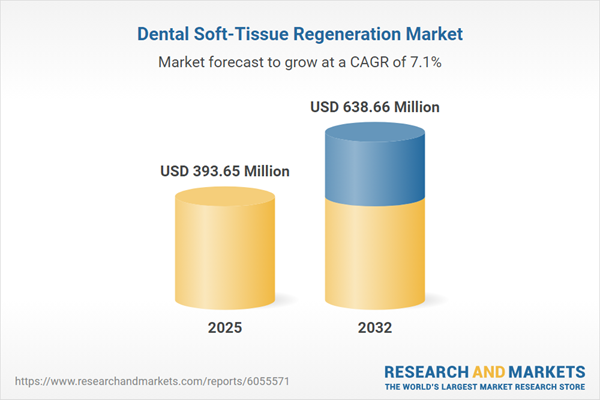
| Estimated Market Value ( USD | $ 393.65 Million |
| Forecasted Market Value ( USD | $ 638.66 Million |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |