Global Medical Device Regulatory Affairs Market - Key Trends & Drivers Summarized
Why Are Regulatory Affairs Becoming Central to Medical Device Commercialization?
In today's rapidly evolving healthcare landscape, regulatory affairs have become a strategic cornerstone in the medical device industry, playing a vital role in ensuring that products meet the strict safety, efficacy, and performance standards required for market approval. As medical devices grow more complex - often integrating software, digital connectivity, and AI - the regulatory approval process has correspondingly become more intricate and time-consuming. Companies are increasingly investing in dedicated regulatory affairs teams or outsourcing expertise to ensure smooth navigation through pre-market submissions, product classifications, risk management protocols, and post-market surveillance requirements.Regulatory bodies such as the U.S. FDA, European Medicines Agency (EMA), Japan's PMDA, and China's NMPA are continuously updating their frameworks to keep pace with innovation. Recent regulations like the EU Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced stricter rules on clinical evaluation, device classification, traceability, and post-market obligations. These developments have transformed regulatory affairs from a backend compliance function into a strategic partner in product development. Companies now integrate regulatory strategy early in the product lifecycle to mitigate the risk of launch delays, market withdrawals, or reputational damage. As the global medical device market expands across new geographies, regulatory harmonization and localization are becoming dual imperatives, requiring in-depth knowledge of both international standards and country-specific nuances.
How Are Technology and Data Shaping the Future of Regulatory Submissions?
Technology is reshaping regulatory affairs, bringing in a wave of digital tools and data-driven systems that enhance efficiency, accuracy, and transparency in managing submissions and compliance. Electronic submissions through platforms like FDA's eSTAR and EMA's EUDAMED are replacing manual documentation, reducing errors, improving timelines, and enabling easier access to regulatory data. Regulatory Information Management Systems (RIMS) are gaining popularity for tracking submission status, managing product registrations across countries, and maintaining regulatory intelligence databases. These systems are particularly valuable for companies operating in multiple jurisdictions with varying document formats, approval timelines, and surveillance requirements.Artificial Intelligence (AI) and machine learning are beginning to find applications in regulatory affairs as well - automating literature searches, analyzing adverse event patterns, and predicting regulatory risks based on historical data. Natural Language Processing (NLP) tools are being used to extract and organize large volumes of regulatory text, enabling teams to generate submission-ready documentation more efficiently. Furthermore, cloud-based platforms are facilitating global collaboration between regulatory teams, clinical experts, and consultants, ensuring alignment throughout the lifecycle of a device. The integration of real-world evidence (RWE) and real-world data (RWD) into regulatory decision-making is also gaining momentum, with agencies beginning to accept these data types for post-market monitoring and, in some cases, approval extensions. These technological advancements are streamlining operations and allowing regulatory affairs professionals to take on more strategic and analytical roles within organizations.
Who's Leading the Charge and How Is Globalization Influencing Regulatory Strategies?
The regulatory affairs market is expanding rapidly across all segments of the medical device industry - from multinational corporations with vast product portfolios to emerging startups bringing disruptive innovations to market. Large medtech companies are building in-house regulatory teams with global expertise to manage extensive pipelines and multiple market entries, while small and mid-sized firms are increasingly turning to specialized regulatory consultants or contract research organizations (CROs) for support. This shift toward outsourcing is especially prevalent in highly regulated markets like the EU and U.S., where navigating submissions such as 510(k), PMA, CE marking, or technical dossiers can be resource-intensive and time-sensitive.Globalization is exerting a strong influence on regulatory strategies, as manufacturers seek access to high-growth markets in Asia-Pacific, Latin America, and the Middle East. These regions are enhancing their regulatory frameworks to match international standards while maintaining local compliance requirements - demanding a nuanced, region-specific approach. For example, China's evolving NMPA approval process now mandates local clinical data for many devices, prompting foreign firms to collaborate with local entities. In parallel, initiatives such as the International Medical Device Regulators Forum (IMDRF) are working toward harmonizing submission formats and terminology to reduce redundancy and facilitate global approvals. In response, companies are restructuring regulatory teams to function as agile, cross-border units capable of adapting to both centralized and decentralized market models.
What's Driving Growth in the Medical Device Regulatory Affairs Market?
The growth in the medical device regulatory affairs market is driven by several interrelated factors linked to technological innovation, regulatory tightening, and expanding global healthcare markets. First and foremost, the increasing complexity of medical devices - especially those involving software as a medical device (SaMD), AI-driven diagnostics, and combination products - necessitates deeper regulatory engagement and proactive strategy development. This complexity is further amplified by the shift from periodic submission-based compliance to continuous lifecycle monitoring, compelling companies to maintain real-time vigilance over safety signals, field performance, and labeling updates.End-use diversification is also contributing to market growth. As non-traditional players such as digital health startups, pharma-biotech firms, and consumer tech companies enter the medical device space, they require specialized regulatory support to navigate unfamiliar regulatory landscapes. Additionally, growing patient demand for transparency and safety, combined with media scrutiny over device recalls or adverse events, is pushing firms to prioritize compliance and risk communication as central to brand credibility. On the operational side, the rise of remote audits, digital submissions, and AI-enabled regulatory technologies is improving submission accuracy and reducing review times - enhancing return on investment and driving further adoption.
Lastly, the global expansion of healthcare systems and the increasing volume of clinical trials involving devices are pushing demand for regulatory professionals with cross-functional expertise. The scarcity of skilled regulatory talent in many markets is further fueling the growth of regulatory service providers and training platforms. Together, these drivers are positioning regulatory affairs not just as a compliance function but as a vital, growth-enabling force in the medical device innovation ecosystem.
Report Scope
The report analyzes the Medical Device Regulatory Affairs market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Service (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Applications, Other Services); Type (Diagnostic, Therapeutic); Service Provider (In-house, Outsource).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Regulatory Consulting segment, which is expected to reach US$7.5 Billion by 2030 with a CAGR of a 9.7%. The Legal Representation segment is also set to grow at 6.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.6 Billion in 2024, and China, forecasted to grow at an impressive 13% CAGR to reach $4.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Medical Device Regulatory Affairs Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Medical Device Regulatory Affairs Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Medical Device Regulatory Affairs Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ALKU, Apotech Consulting, BCG (Boston Consulting Group), Celegence, Certara and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Medical Device Regulatory Affairs market report include:
- ALKU
- Apotech Consulting
- BCG (Boston Consulting Group)
- Celegence
- Certara
- Elexes
- Emergo by UL
- Fang Consulting
- Freyr Solutions
- ICON plc
- MCRA
- MWA Consulting
- NAMSA
- Operon Strategist
- QES Medical
- Rook Quality Systems
- RQM+
- Sigma Management Systems
- Umbrex
- Veranex
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ALKU
- Apotech Consulting
- BCG (Boston Consulting Group)
- Celegence
- Certara
- Elexes
- Emergo by UL
- Fang Consulting
- Freyr Solutions
- ICON plc
- MCRA
- MWA Consulting
- NAMSA
- Operon Strategist
- QES Medical
- Rook Quality Systems
- RQM+
- Sigma Management Systems
- Umbrex
- Veranex
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 380 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
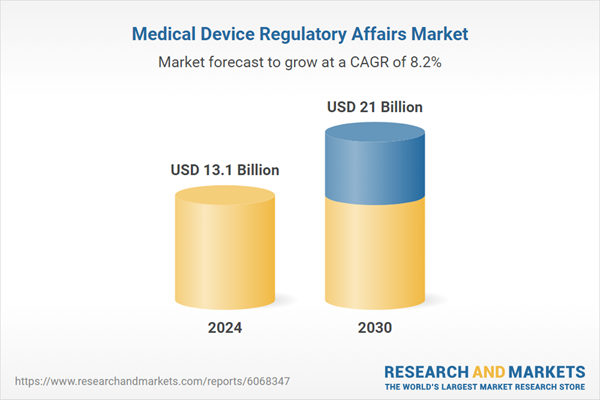
| Estimated Market Value ( USD | $ 13.1 Billion |
| Forecasted Market Value ( USD | $ 21 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |