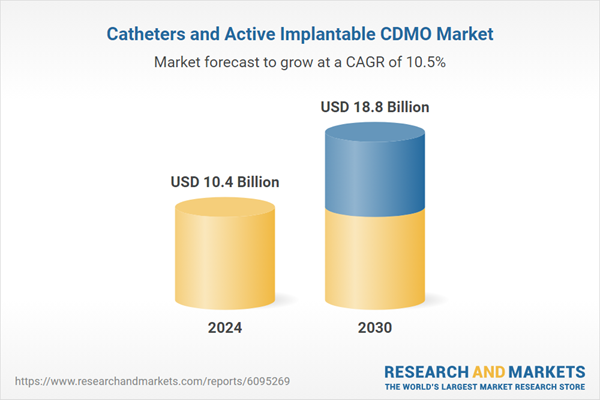
Global 'Catheters and Active Implantable CDMO' Market - Key Trends & Drivers Summarized
Why Is There a Surge in Demand for CDMO Services in Catheters and Active Implantables?
The market for catheters and active implantable devices has seen a significant uptick, propelled by a fusion of complex patient demands and rapidly evolving medical technologies. At the heart of this trend lies the growing reliance on Contract Development and Manufacturing Organizations (CDMOs) to deliver highly specialized solutions across cardiovascular, neurovascular, urology, and electrophysiology applications. As medical device OEMs (Original Equipment Manufacturers) face mounting regulatory hurdles and the need for faster time-to-market, CDMOs have emerged as vital strategic partners. These specialized firms bring in-depth expertise in regulatory compliance, precision engineering, and clinical-grade manufacturing, enabling OEMs to focus on core R&D and commercialization. In catheter development, CDMOs are increasingly being tasked with managing everything from raw material selection and extrusion to final sterilization and packaging. The growing complexity of active implantables - such as cardiac rhythm management devices, neurostimulators, and infusion pumps - requires multidomain knowledge in microelectronics, software integration, and biocompatible materials, all of which high-end CDMOs are well-equipped to handle.How Are Technological Innovations Transforming Product Development Pipelines?
Cutting-edge technological integration is radically reshaping the CDMO landscape for catheters and active implantables. Innovations in material science - such as the use of ultra-thin polymers, hydrophilic coatings, and bioresorbable composites - are enabling the development of catheters that are safer, more comfortable, and more functional. Similarly, in the active implantables domain, miniaturization and power efficiency are guiding principles driving next-gen devices. CDMOs are at the forefront of incorporating wireless telemetry, rechargeable batteries, and software-controlled functionalities in implantable devices. Additionally, 3D printing is being increasingly adopted for prototyping complex catheter tip geometries and housings for implantables. The convergence of IoT (Internet of Things) and MedTech is also redefining expectations, with smart catheters capable of real-time data transmission and implantables that can self-monitor and adjust therapy parameters. CDMOs with robust electronics integration and software validation capabilities are becoming indispensable partners in this innovation-driven ecosystem. Their ability to scale manufacturing of such high-precision, regulatory-bound devices is a key enabler of market acceleration.What Market Pressures Are Shaping CDMO Engagement Strategies?
A highly competitive healthcare environment, shrinking product lifecycles, and increasing cost pressures are reshaping how OEMs engage with CDMOs. Outsourcing is no longer limited to cost-efficiency - it has become a strategic move to gain competitive advantage. CDMOs are now expected to offer integrated end-to-end services that span early design and prototyping, regulatory documentation, clinical trial support, and commercial-scale production. Moreover, with tightening regulatory frameworks by bodies such as the FDA, MDR (EU), and NMPA (China), CDMOs must ensure global compliance and traceability across the product life cycle. This regulatory complexity is pushing OEMs to seek CDMOs with established quality systems (such as ISO 13485 certification), in-house testing labs, and digital traceability platforms. Consolidation is also being witnessed within the CDMO space itself, with larger players acquiring niche technology firms to broaden capabilities across catheter and implantable verticals. This trend enables bundled service offerings, faster development timelines, and streamlined tech transfers - all critical factors for OEMs racing to commercialize next-gen medical devices.The Growth In The Catheters And Active Implantable CDMO Market Is Driven By Several Factors……..
The growth in the catheters and active implantable CDMO market is driven by several factors rooted in device complexity, end-user needs, and strategic shifts within the MedTech industry. First, the rising prevalence of chronic conditions such as cardiovascular disease, neurological disorders, and diabetes is increasing demand for both diagnostic and therapeutic minimally invasive devices, expanding the need for catheter and implantable development. Second, hospitals and clinicians are increasingly favoring miniaturized, multifunctional devices that demand high levels of design intricacy and precision - capabilities typically concentrated within specialized CDMOs. Third, OEMs are offloading non-core operations such as manufacturing and regulatory submissions to CDMOs in order to focus on clinical innovation and market expansion. Furthermore, the medical device market's push towards personalization - such as patient-specific catheter sizing or programmable implants - is increasing reliance on CDMOs with rapid prototyping and short-run manufacturing expertise. Geographic shifts in healthcare investments - particularly in Asia-Pacific, where governments are incentivizing local manufacturing - are also fueling regional CDMO expansions. Lastly, increased venture capital funding for MedTech startups is driving outsourcing from early development stages, creating sustained growth opportunities for CDMOs across the value chain.Report Scope
The report analyzes the Catheters and Active Implantable CDMO market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Service Category (Catheters, Active Implantable Devices, Neuromodulation Devices, Heart Failure Devices, Urology Implantable Devices); End-Use (Medical Device OEM Companies End-Use, Academic & Research Institutes End-Use, Government Agencies End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Catheters segment, which is expected to reach US$7.8 Billion by 2030 with a CAGR of a 12%. The Active Implantable Devices segment is also set to grow at 9.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.8 Billion in 2024, and China, forecasted to grow at an impressive 14.5% CAGR to reach $3.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Catheters and Active Implantable CDMO Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Catheters and Active Implantable CDMO Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Catheters and Active Implantable CDMO Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abcam Corporation, AK Scientific Inc., Amadis Chemical Co., Ltd., Anisyn, Inc., Bio Vision Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Catheters and Active Implantable CDMO market report include:
- Abbott Laboratories
- Alvimedica
- Biomerics
- Biotronik
- Boston Scientific
- Catalent
- Cirtec Medical
- Cochlear Limited
- ConvaTec Group
- Freudenberg Medical
- Heraeus Group
- Integer Holdings
- LeMaitre Vascular
- Medtronic
- MicroPort
- OrbusNeich
- Recipharm
- Smith & Nephew
- Teleflex
- Vetter Pharma
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Alvimedica
- Biomerics
- Biotronik
- Boston Scientific
- Catalent
- Cirtec Medical
- Cochlear Limited
- ConvaTec Group
- Freudenberg Medical
- Heraeus Group
- Integer Holdings
- LeMaitre Vascular
- Medtronic
- MicroPort
- OrbusNeich
- Recipharm
- Smith & Nephew
- Teleflex
- Vetter Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 277 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 10.4 Billion |
| Forecasted Market Value ( USD | $ 18.8 Billion |
| Compound Annual Growth Rate | 10.5% |
| Regions Covered | Global |