Global Invasive Ductal Carcinoma Treatment Market - Key Trends & Drivers Summarized
How Is the Treatment Landscape for Invasive Ductal Carcinoma Evolving?
The invasive ductal carcinoma (IDC) treatment market is rapidly evolving due to advancements in targeted therapies, precision medicine, and immunotherapy approaches. Invasive ductal carcinoma, the most common form of breast cancer, accounts for approximately 80% of all diagnosed cases and originates in the milk ducts before spreading to surrounding breast tissues. Treatment strategies for IDC depend on tumor size, hormone receptor status (HR+/-), HER2 status, and the presence of genetic mutations, making personalized therapy a crucial component of modern treatment protocols.The rising prevalence of breast cancer, increasing adoption of minimally invasive surgical techniques, and expansion of innovative systemic therapies are driving continuous advancements in IDC treatment. With the integration of AI-driven diagnostics, genomic profiling, and liquid biopsy technologies, oncologists can now tailor treatments to individual patients, improving outcomes and reducing unnecessary toxicity.
Additionally, government initiatives, increasing research funding, and collaborations between biotech firms and pharmaceutical companies are accelerating the development of next-generation therapies, including antibody-drug conjugates (ADCs), PARP inhibitors, and bispecific antibodies for IDC treatment.
What Are the Key Trends Driving Innovations in IDC Treatment?
One of the most significant trends in IDC treatment is the rise of targeted therapy and immunotherapy. With growing insights into molecular subtyping and genetic mutations in breast cancer, oncologists are shifting away from traditional chemotherapy and focusing on precision-targeted treatments that enhance efficacy while minimizing side effects.For hormone receptor-positive (HR+) IDC, selective estrogen receptor modulators (SERMs), aromatase inhibitors (AIs), and CDK4/6 inhibitors (such as Palbociclib, Ribociclib, and Abemaciclib) are becoming standard of care, preventing tumor growth by blocking estrogen-driven cancer proliferation.
For HER2-positive IDC, monoclonal antibodies such as Trastuzumab (Herceptin), Pertuzumab (Perjeta), and the antibody-drug conjugate Trastuzumab-Deruxtecan (Enhertu) have significantly improved survival rates by specifically targeting HER2-overexpressing cancer cells. The development of next-generation HER2 inhibitors is expected to expand treatment options for HER2+ IDC patients, particularly those with resistance to current therapies.
Another emerging trend is the increasing use of immune checkpoint inhibitors (ICIs) in triple-negative breast cancer (TNBC), a particularly aggressive IDC subtype that lacks estrogen, progesterone, and HER2 receptors. Atezolizumab (Tecentriq) and Pembrolizumab (Keytruda), combined with chemotherapy, have shown promising results in improving progression-free survival (PFS) and overall survival (OS) in PD-L1-positive TNBC patients.
The expansion of PARP inhibitors (such as Olaparib and Talazoparib) for BRCA-mutated IDC cases is another key development, offering a novel approach to targeting DNA damage repair pathways in breast cancer cells. These agents are particularly effective in patients with hereditary BRCA1/2 mutations, reducing the risk of recurrence and improving treatment response rates.
Additionally, neoadjuvant therapy (pre-surgical systemic treatment) is gaining traction, allowing tumor size reduction before surgery, enabling breast-conserving procedures (lumpectomy) over mastectomy. The combination of chemotherapy, targeted agents, and immune-based therapies in the neoadjuvant setting has led to higher pathological complete response (pCR) rates, improving long-term survival outcomes.
What Challenges Are Impacting the IDC Treatment Market?
Despite significant progress in IDC treatment, several challenges remain, affecting access, affordability, and treatment efficacy. One of the primary concerns is drug resistance and disease recurrence, particularly in metastatic IDC cases. Patients with advanced-stage IDC often develop resistance to endocrine therapy, HER2-targeted agents, and chemotherapy, requiring novel combination strategies and next-generation inhibitors to overcome treatment resistance mechanisms.Financial burden and healthcare disparities are also major challenges. Targeted therapies and immunotherapies can be prohibitively expensive, limiting access to low- and middle-income populations. Lack of insurance coverage, high out-of-pocket costs, and disparities in healthcare infrastructure hinder equitable access to life-saving treatments, particularly in developing countries.
Another challenge is treatment-related toxicity and side effects. While targeted therapies and immunotherapies offer better specificity than traditional chemotherapy, many patients experience adverse effects such as cardiotoxicity (HER2 inhibitors), neutropenia (CDK4/6 inhibitors), and immune-related toxicities (ICIs). Optimizing supportive care strategies, dose adjustments, and biomarker-driven patient selection are crucial to minimizing toxicity while maintaining therapeutic efficacy.
The complexity of IDC subtyping and biomarker testing is also a barrier. Accurate molecular profiling and genetic testing (e.g., BRCA, PIK3CA, PD-L1, HER2-low status) are essential for personalizing treatment decisions. However, limited access to genomic testing and lack of standardization in biomarker-driven treatment algorithms can delay the implementation of precision oncology approaches in clinical practice.
What Factors Are Driving the Growth of the IDC Treatment Market?
The growth in the IDC treatment market is driven by advancements in precision medicine, increasing breast cancer incidence, and rising awareness about early diagnosis and treatment options. One of the key drivers is the expanding pipeline of novel targeted agents and biologics, which are continuously improving survival rates and reducing the toxicity burden of traditional chemotherapy.The integration of liquid biopsy, AI-driven diagnostics, and next-generation sequencing (NGS) is also accelerating early detection, minimal residual disease (MRD) monitoring, and treatment response assessment, allowing for timely intervention and adaptive therapy adjustments.
Another major growth factor is government initiatives and public health campaigns promoting breast cancer screening, genetic counseling, and awareness programs. Many countries are investing in national screening programs, subsidizing biomarker testing, and providing patient assistance programs for expensive cancer treatments.
The rise of combination therapies and novel drug delivery platforms is also shaping market expansion. Bi-specific antibodies, antibody-drug conjugates (ADCs), and next-generation small molecule inhibitors are enhancing treatment efficacy while minimizing off-target toxicity, making them promising candidates for future IDC treatment regimens.
Additionally, the increasing role of patient-centric and supportive care strategies, including personalized nutrition, psychological support, and integrative oncology, is improving overall treatment experiences and quality of life for IDC patients.
Report Scope
The report analyzes the Invasive Ductal Carcinoma Treatment market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Therapy (Targeted Therapy, Hormonal Therapy, Chemotherapy, Immunotherapy); Type (Hormone Receptor, HER2+); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Targeted Therapy segment, which is expected to reach US$11.0 Billion by 2030 with a CAGR of a 7.1%. The Hormonal Therapy segment is also set to grow at 8.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $4.9 Billion in 2024, and China, forecasted to grow at an impressive 11.6% CAGR to reach $5.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Invasive Ductal Carcinoma Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Invasive Ductal Carcinoma Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Invasive Ductal Carcinoma Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Invasive Ductal Carcinoma Treatment market report include:
- AbbVie
- Amgen
- Astellas Pharma Inc.
- AstraZeneca
- Bayer
- Biogen
- Bristol-Myers Squibb
- Eli Lilly and Company
- Exact Sciences Corp.
- Genentech (Roche)
- GSK
- Johnson & Johnson
- Merck & Co.
- Novartis
- Oncomatryx
- Pfizer
- Roche
- Sanofi
- SynDevRx
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie
- Amgen
- Astellas Pharma Inc.
- AstraZeneca
- Bayer
- Biogen
- Bristol-Myers Squibb
- Eli Lilly and Company
- Exact Sciences Corp.
- Genentech (Roche)
- GSK
- Johnson & Johnson
- Merck & Co.
- Novartis
- Oncomatryx
- Pfizer
- Roche
- Sanofi
- SynDevRx
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 375 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
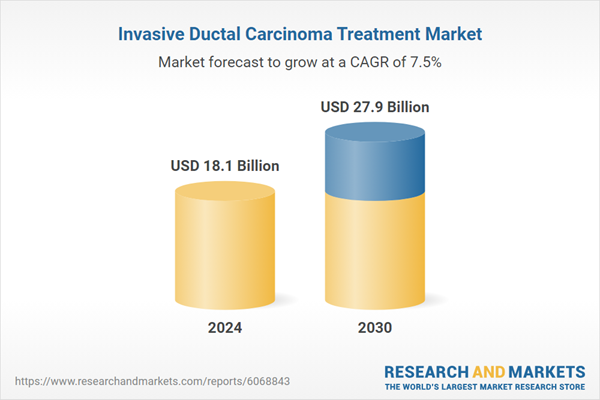
| Estimated Market Value ( USD | $ 18.1 Billion |
| Forecasted Market Value ( USD | $ 27.9 Billion |
| Compound Annual Growth Rate | 7.5% |
| Regions Covered | Global |