Global Lipid Nanoparticle Raw Materials Market - Key Trends & Drivers Summarized
Why Are Lipid Nanoparticles Gaining Prominence in Drug Delivery and Biotechnology?
Lipid nanoparticles (LNPs) have revolutionized drug delivery and biotechnology by serving as highly efficient carriers for mRNA-based vaccines, gene therapies, and targeted drug delivery systems. Their ability to encapsulate and protect fragile biomolecules, such as nucleic acids and proteins, has positioned them as a critical component in modern pharmaceutical formulations. The COVID-19 pandemic accelerated the adoption of lipid nanoparticles, particularly with the success of mRNA vaccines from Pfizer-BioNTech and Moderna, which relied heavily on LNP-based delivery mechanisms. Unlike traditional drug delivery vehicles, LNPs provide enhanced stability, improved bioavailability, and targeted release, reducing systemic toxicity and improving therapeutic efficacy. The increasing focus on precision medicine and personalized drug formulations has further fueled research and development in LNP technology. Additionally, advancements in nanotechnology and lipid chemistry have enabled the production of highly specialized LNP formulations tailored for diverse therapeutic applications, including cancer therapy, rare genetic disorders, and infectious diseases. The pharmaceutical industry's growing reliance on LNPs has driven a surge in demand for high-quality raw materials, such as ionizable lipids, phospholipids, cholesterol, and PEGylated lipids, which are essential for optimizing LNP formulations.What Are the Key Raw Materials Driving Lipid Nanoparticle Innovations?
The lipid nanoparticle market relies on a precise blend of raw materials, each playing a crucial role in maintaining the stability, efficiency, and biocompatibility of the final formulation. Ionizable lipids are a critical component, allowing LNPs to encapsulate nucleic acids efficiently and release them upon cellular uptake. The continuous optimization of ionizable lipids has improved the effectiveness of mRNA vaccines and gene therapies, reducing the required dosage and minimizing immune responses. Phospholipids act as structural stabilizers, ensuring the integrity of LNPs and facilitating their interaction with biological membranes. Cholesterol, another essential component, enhances lipid bilayer rigidity, improving nanoparticle stability and circulation time in the bloodstream. PEGylated lipids (polyethylene glycol lipids) reduce immune recognition, preventing rapid clearance from the body and extending the half-life of LNP-based drugs. Additionally, researchers are exploring next-generation lipids, such as biodegradable lipids and stimuli-responsive lipids, to enhance delivery efficiency and minimize long-term accumulation in tissues. The growing demand for high-purity, pharmaceutical-grade lipids has led to the expansion of specialized suppliers and manufacturers capable of meeting the stringent regulatory requirements of the pharmaceutical industry.How Are Technological Advancements Shaping Lipid Nanoparticle Manufacturing?
The production of lipid nanoparticles has undergone significant technological advancements, ensuring scalable, reproducible, and cost-effective manufacturing processes. One of the most notable innovations is microfluidic-based LNP synthesis, which enables precise control over nanoparticle size, uniformity, and encapsulation efficiency. This technique allows for high-throughput production while minimizing batch-to-batch variability, making it ideal for large-scale vaccine and drug manufacturing. Additionally, AI-driven formulation design and computational modeling are accelerating the discovery of novel lipid structures optimized for specific drug delivery applications. The integration of machine learning algorithms into lipid screening processes has significantly reduced formulation development timelines, expediting the commercialization of new LNP-based therapeutics. Moreover, advancements in lipid purification and characterization techniques, such as high-performance liquid chromatography (HPLC) and mass spectrometry, have improved the consistency and quality of raw materials, ensuring regulatory compliance and product safety. The increasing adoption of continuous manufacturing techniques has also streamlined the production of lipid nanoparticles, reducing costs and enhancing efficiency. Another major development in the field is the emergence of biomanufacturing approaches that utilize enzymatic synthesis of lipids, offering a sustainable and eco-friendly alternative to traditional chemical synthesis. These technological advancements are playing a crucial role in meeting the rising demand for lipid nanoparticles across multiple therapeutic areas.What Are the Key Market Drivers Fueling the Demand for Lipid Nanoparticle Raw Materials?
The growth in the lipid nanoparticle raw materials market is driven by several factors, including the increasing adoption of mRNA-based therapeutics, the expansion of gene editing technologies, and the rising prevalence of chronic diseases requiring targeted drug delivery. The success of LNP-based COVID-19 vaccines has set a precedent for the rapid development and approval of next-generation LNP-based therapies, accelerating demand for high-quality lipid raw materials. The growing pipeline of RNA-based treatments for conditions such as cancer, rare genetic disorders, and metabolic diseases has further increased reliance on lipid nanoparticle systems. Additionally, the expansion of cell and gene therapy applications, including CRISPR-Cas9-based gene editing, has fueled the need for highly efficient and biocompatible lipid carriers. The increasing investment in nanomedicine research by pharmaceutical companies and academic institutions has driven continuous improvements in LNP formulations, leading to more effective and safer therapies. Regulatory support for advanced drug delivery systems has also played a key role in market growth, with agencies such as the FDA and EMA providing streamlined approval pathways for LNP-based therapeutics. Furthermore, the rise of contract development and manufacturing organizations (CDMOs) specializing in LNP production has expanded access to high-quality lipid raw materials, enabling pharmaceutical companies to scale up production efficiently. The growing focus on sustainability and green chemistry in drug manufacturing has also led to increased research into biodegradable and naturally derived lipids, aligning with industry efforts to reduce environmental impact. Looking ahead, the continued evolution of lipid-based drug delivery systems, combined with advancements in synthetic biology and nanomedicine, is expected to drive further innovation in the lipid nanoparticle raw materials market, cementing its role as a cornerstone of modern pharmaceutical development.Report Scope
The report analyzes the Lipid Nanoparticle Raw Materials market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Ionizable lipids, Reagents, Other Raw Materials); Disease Indication (Cancer, Infectious Diseases, Blood Diseases, Others); End-Use (Pharma & Biotech Companies, Academic & Research Institutes, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 32 companies featured in this Lipid Nanoparticle Raw Materials market report include -
- Acuitas Therapeutics
- Avanti Polar Lipids (Croda International plc)
- Biopharma PEG Scientific Inc.
- BIOVECTRA
- BroadPharm
- Cayman Chemical
- CordenPharma International
- Creative Biolabs
- Curapath
- Echelon Biosciences
- Evonik Industries AG
- Gattefossé
- Lipoid GmbH
- MedKoo Biosciences, Inc.
- Merck KGaA
- Nanocs, Inc.
- NOF Corporation
- Polysciences, Inc.
- Precision NanoSystems
- Tebubio
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Ionizable Lipids segment, which is expected to reach US$180.9 Million by 2030 with a CAGR of a 4.2%. The Reagents segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $62.7 Million in 2024, and China, forecasted to grow at an impressive 8% CAGR to reach $61.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lipid Nanoparticle Raw Materials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lipid Nanoparticle Raw Materials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lipid Nanoparticle Raw Materials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Acuitas Therapeutics, Avanti Polar Lipids (Croda International plc), Biopharma PEG Scientific Inc., BIOVECTRA, BroadPharm and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 32 Featured):
- Acuitas Therapeutics
- Avanti Polar Lipids (Croda International plc)
- Biopharma PEG Scientific Inc.
- BIOVECTRA
- BroadPharm
- Cayman Chemical
- CordenPharma International
- Creative Biolabs
- Curapath
- Echelon Biosciences
- Evonik Industries AG
- Gattefossé
- Lipoid GmbH
- MedKoo Biosciences, Inc.
- Merck KGaA
- Nanocs, Inc.
- NOF Corporation
- Polysciences, Inc.
- Precision NanoSystems
- Tebubio
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acuitas Therapeutics
- Avanti Polar Lipids (Croda International plc)
- Biopharma PEG Scientific Inc.
- BIOVECTRA
- BroadPharm
- Cayman Chemical
- CordenPharma International
- Creative Biolabs
- Curapath
- Echelon Biosciences
- Evonik Industries AG
- Gattefossé
- Lipoid GmbH
- MedKoo Biosciences, Inc.
- Merck KGaA
- Nanocs, Inc.
- NOF Corporation
- Polysciences, Inc.
- Precision NanoSystems
- Tebubio
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 368 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
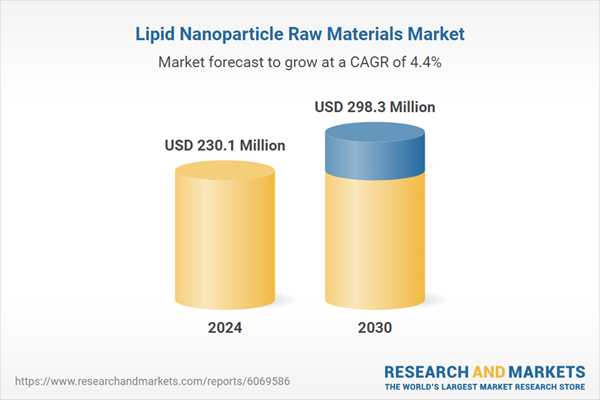
| Estimated Market Value ( USD | $ 230.1 Million |
| Forecasted Market Value ( USD | $ 298.3 Million |
| Compound Annual Growth Rate | 4.4% |
| Regions Covered | Global |