Global Lipid Nanoparticle Manufacturing Market - Key Trends & Drivers Summarized
Why Are Lipid Nanoparticles at the Center of a New Era in Drug Delivery and Therapeutics?
Lipid nanoparticles have become a foundational technology in modern drug delivery systems, driven by their ability to encapsulate and protect fragile therapeutic agents such as nucleic acids, peptides, and small molecules. Their emergence as a vital component in the delivery of mRNA vaccines, particularly during the global COVID-19 vaccination effort, has brought widespread attention to their biomedical potential. Lipid nanoparticles serve as non-viral vectors that enhance the stability, bioavailability, and cellular uptake of drugs, making them especially valuable in therapies that require targeted delivery and controlled release. Their capacity to carry genetic materials such as mRNA and siRNA into cells without causing significant immunogenicity or toxicity positions them as indispensable tools in gene therapy, cancer treatment, and next-generation vaccines. Researchers are increasingly relying on lipid nanoparticle platforms to overcome the delivery challenges posed by hydrophilic, large-molecule drugs that degrade quickly in the bloodstream. The ability of these particles to fuse with cell membranes and release their payload in a controlled manner enhances therapeutic precision and minimizes side effects. As personalized medicine becomes more prominent and complex biologics are developed, lipid nanoparticles are expected to play an even greater role in ensuring therapeutic efficacy. The expanding pipeline of nucleic acid-based therapeutics, including mRNA cancer vaccines and gene editing treatments, underscores the critical importance of scalable and consistent lipid nanoparticle manufacturing technologies.How Are Technological Advancements Transforming Lipid Nanoparticle Manufacturing Capabilities?
Lipid nanoparticle manufacturing has evolved rapidly in recent years, thanks to significant advances in formulation science, microfluidics, and process engineering. The transition from traditional solvent-based methods to precise, scalable microfluidic mixing techniques has dramatically improved particle uniformity, reproducibility, and encapsulation efficiency. These advancements are particularly crucial for clinical-grade lipid nanoparticles, where tight control over particle size, polydispersity, and surface charge is essential to ensure consistent performance and regulatory compliance. The development of modular and continuous manufacturing systems has allowed for more efficient production at scale, enabling rapid response to demand surges such as those experienced during the COVID-19 pandemic. Automation and real-time monitoring technologies are increasingly being integrated into lipid nanoparticle production lines, improving quality assurance and reducing batch-to-batch variability. Innovations in lipid chemistry are also expanding the functionality of nanoparticles, with synthetic and ionizable lipids being engineered to enhance drug loading, biocompatibility, and endosomal escape. Furthermore, the integration of artificial intelligence and machine learning into formulation development is accelerating the discovery of optimal lipid combinations and process conditions. This high level of innovation is being supported by robust intellectual property activity and partnerships between pharmaceutical companies, biotech startups, and academic research centers. With increasing demand for specialized formulations in oncology, rare diseases, and infectious diseases, manufacturers are investing in flexible facilities capable of producing a wide range of nanoparticle compositions under current Good Manufacturing Practices (cGMP). These advancements are reshaping the lipid nanoparticle manufacturing landscape into a dynamic, high-precision domain capable of supporting the next wave of biotherapeutic breakthroughs.What Market and Regulatory Trends Are Shaping the Evolution of Lipid Nanoparticle Production?
The lipid nanoparticle manufacturing market is being influenced by a complex interplay of regulatory requirements, biopharmaceutical demand, and investment trends, all of which are shaping how facilities operate and innovate. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have begun to establish clearer frameworks for evaluating the safety, efficacy, and quality control of lipid nanoparticle-based products, particularly those used in nucleic acid therapies. These evolving standards are prompting manufacturers to invest heavily in analytical tools and validation systems to meet stringent compliance expectations. At the same time, global demand for mRNA-based therapies and vaccines continues to rise, encouraging both established pharmaceutical firms and contract development and manufacturing organizations (CDMOs) to scale up production capabilities. Emerging markets are also entering the scene, with countries in Asia-Pacific, Latin America, and the Middle East beginning to invest in domestic nanoparticle manufacturing capacity to reduce dependency on external supply chains. Venture capital and government funding are flowing into lipid nanoparticle startups and technology developers, driven by the broader recognition of their strategic role in future pandemic preparedness and therapeutic innovation. Environmental and sustainability concerns are also beginning to influence manufacturing practices, pushing for greener solvents and more energy-efficient production methods. Intellectual property disputes and licensing agreements are another factor shaping the competitive landscape, as companies race to secure exclusive rights to proprietary lipid compositions and manufacturing protocols. As the market matures, strategic collaborations between biotech companies, academic institutions, and CMOs are becoming increasingly important to accelerate time-to-market and share the technical burden of scaling complex nanoparticle systems.What Forces Are Driving the Accelerated Growth of the Lipid Nanoparticle Manufacturing Market?
The growth in the lipid nanoparticle manufacturing market is driven by several interrelated factors closely aligned with the rise of genetic medicine, increased R&D investments, and evolving therapeutic strategies. A primary growth driver is the expanding development pipeline of mRNA vaccines and gene therapies, which require advanced delivery systems that only lipid nanoparticles can provide efficiently and safely. The success of mRNA COVID-19 vaccines has proven the scalability and clinical viability of lipid nanoparticle delivery platforms, encouraging broader adoption in other therapeutic areas such as oncology, rare genetic disorders, and chronic diseases. Biopharmaceutical companies are now prioritizing lipid nanoparticle technologies in their strategic roadmaps, leading to increased collaborations with contract manufacturers and suppliers of specialized lipids. Another key driver is the heightened global preparedness for future pandemics and emerging infectious diseases, prompting governments and healthcare agencies to stockpile mRNA-based vaccines and invest in rapid-response manufacturing capabilities. The rise of personalized medicine, which demands flexible, small-batch production of tailored therapies, is also favoring modular lipid nanoparticle manufacturing systems that can adapt to varying drug profiles and formulations. Additionally, the globalization of biotech innovation is expanding demand for localized, compliant production facilities that can serve regional markets without delays or logistical bottlenecks. Technological advancements in lipid synthesis, microfluidic device fabrication, and real-time quality control are making it more feasible to produce high-quality nanoparticles at industrial scale. Finally, regulatory incentives, expedited approval pathways for innovative delivery systems, and increased public and private funding are all reinforcing the momentum behind lipid nanoparticle manufacturing. Together, these forces are driving a sustained and accelerating growth curve for this highly specialized and strategically critical segment of the pharmaceutical industry.Report Scope
The report analyzes the Lipid Nanoparticle Manufacturing market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: LNP Type (Solid Lipid Nanoparticles, Nanostructured Lipid Carriers); Therapeutic Area (Infectious Diseases Therapeutic Area, Rare Diseases Therapeutic Area, Oncological Disorders Therapeutic Area, Neurodegenerative Disorders Therapeutic Area, Other Therapeutic Areas); End-Use (Pharma & Biotech Companies End-Use, Academic & Research Institutes End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Solid Lipid Nanoparticles segment, which is expected to reach US$880.3 Million by 2030 with a CAGR of a 7.3%. The Nanostructured Lipid Carriers segment is also set to grow at 11.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $237.6 Million in 2024, and China, forecasted to grow at an impressive 11.8% CAGR to reach $285.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lipid Nanoparticle Manufacturing Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lipid Nanoparticle Manufacturing Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lipid Nanoparticle Manufacturing Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Activa Naturals Inc., Applied Food Sciences, Inc., Biofinest, DIRTEA, Double Wood Supplements and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Lipid Nanoparticle Manufacturing market report include:
- Acuitas Therapeutics
- Ardena Holding NV
- Ascendia Pharmaceuticals, Inc.
- Avanti Polar Lipids (Croda Intl. Plc)
- BIOVECTRA
- Catalent, Inc.
- Cayman Chemical
- CordenPharma
- Creative Biolabs
- Curia Global, Inc.
- Curapath
- eTheRNA immunotherapies
- EUROAPI
- Evonik Industries AG
- FUJIFILM Corporation
- Gattefossé
- Genevant Sciences Corporation
- Lipoid GmbH
- Merck KGaA
- NOF CORPORATION
- Precision NanoSystems (Cytiva)
- Recipharm AB
- ST Pharm Co Ltd
- Thermo Fisher Scientific
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Acuitas Therapeutics
- Ardena Holding NV
- Ascendia Pharmaceuticals, Inc.
- Avanti Polar Lipids (Croda Intl. Plc)
- BIOVECTRA
- Catalent, Inc.
- Cayman Chemical
- CordenPharma
- Creative Biolabs
- Curia Global, Inc.
- Curapath
- eTheRNA immunotherapies
- EUROAPI
- Evonik Industries AG
- FUJIFILM Corporation
- Gattefossé
- Genevant Sciences Corporation
- Lipoid GmbH
- Merck KGaA
- NOF CORPORATION
- Precision NanoSystems (Cytiva)
- Recipharm AB
- ST Pharm Co Ltd
- Thermo Fisher Scientific
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 372 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
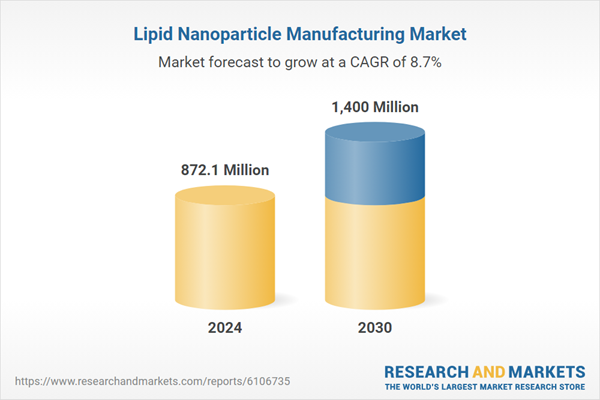
| Estimated Market Value ( USD | $ 872.1 Million |
| Forecasted Market Value ( USD | $ 1400 Million |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |