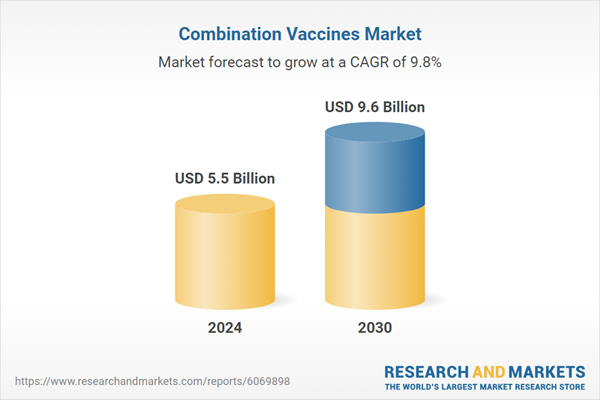
Global Combination Vaccines Market - Key Trends & Drivers Summarized
What Are Combination Vaccines and How Are They Revolutionizing Immunization Programs?
Combination vaccines are advanced immunization formulations that protect against multiple infectious diseases through a single injection. These vaccines are designed to deliver antigens from different pathogens in a single dose, reducing the number of injections required for immunization while maintaining strong immune responses. Common combination vaccines include DTP (diphtheria, tetanus, and pertussis), MMR (measles, mumps, and rubella), and pentavalent vaccines that protect against five diseases, including hepatitis B and Haemophilus influenzae type B (Hib).The increasing adoption of combination vaccines is transforming global immunization programs by improving compliance, reducing logistical challenges, and enhancing overall vaccine coverage. By minimizing the number of injections, combination vaccines help streamline pediatric and adult vaccination schedules, particularly in regions where access to healthcare is limited. As governments and health organizations prioritize disease prevention and outbreak control, combination vaccines are emerging as a key tool in global vaccination strategies, ensuring effective protection against multiple diseases with fewer healthcare visits.
How Are Advancements in Vaccine Technology Enhancing Combination Vaccines?
The development of next-generation combination vaccines has been fueled by advancements in biotechnology, recombinant DNA technology, and adjuvant formulation. Modern combination vaccines utilize recombinant protein subunits, viral vector platforms, and synthetic peptides to enhance immune response without compromising safety or efficacy. Additionally, advancements in antigen stabilization have enabled the creation of thermostable vaccines, reducing the need for stringent cold chain storage and expanding access to immunization in remote areas.Improved adjuvant systems are also enhancing the immunogenicity of combination vaccines, ensuring long-lasting protection while minimizing reactogenicity. Innovations such as mRNA-based combination vaccines are being explored to provide more flexible and rapidly adaptable solutions against emerging infectious diseases. Furthermore, needle-free delivery systems and microneedle patches are being integrated into combination vaccine formulations to improve patient compliance and ease of administration. As research progresses, combination vaccines are expected to play a crucial role in pandemic preparedness, personalized immunization programs, and broader public health initiatives.
Which Diseases and Populations Are Driving the Demand for Combination Vaccines?
The pediatric population represents the largest consumer base for combination vaccines, as childhood immunization programs rely heavily on multi-antigen formulations to provide early protection against life-threatening infections. National immunization schedules worldwide prioritize combination vaccines such as the DTP-Hib-HepB pentavalent vaccine to ensure comprehensive disease prevention with minimal injections. Additionally, adolescent and adult populations are increasingly receiving combination vaccines for diseases such as HPV (human papillomavirus), influenza, and pneumococcal infections.The demand for combination vaccines has surged in response to the growing threat of emerging and re-emerging infectious diseases. Travel and military vaccination programs have also contributed to market expansion, as combination vaccines provide protection against multiple pathogens common in specific geographic regions. Additionally, the increasing incidence of antibiotic-resistant bacterial infections has reinforced the importance of preventive immunization, further driving investment in combination vaccine research and development. As global health organizations continue to emphasize disease eradication and immunization equity, combination vaccines are expected to play an integral role in achieving high vaccination coverage across diverse populations.
What Is Driving the Growth of the Combination Vaccines Market?
The growth in the combination vaccines market is driven by several factors, including advancements in vaccine development, rising global immunization initiatives, increasing demand for pediatric and adult immunization programs, and the need for cost-effective disease prevention strategies. Government agencies, such as the World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC), are promoting combination vaccines as part of routine immunization schedules to reduce vaccine-preventable diseases and improve public health outcomes.The expansion of research collaborations and funding for vaccine innovation has accelerated the development of new multi-antigen formulations, further strengthening market growth. Additionally, the rise of infectious disease outbreaks and pandemic preparedness efforts have highlighted the importance of combination vaccines in rapid immunization responses. Increasing investments in vaccine manufacturing, enhanced supply chain infrastructure, and regulatory approvals for novel combination vaccines are further driving market expansion. With continued technological advancements and the increasing emphasis on global immunization coverage, the combination vaccines market is expected to witness sustained growth, transforming the future of preventive healthcare and infectious disease control.
Report Scope
The report analyzes the Combination Vaccines market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Age Group (Pediatric Age Group, Adult Age Group); Technology (Conjugate Technology, Live Technology, Recombinant Technology, Inactivated Technology, Toxoid Technology, Other Technologies); End-Use (Hospitals End-Use, Clinics End-Use, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pediatric Age Group segment, which is expected to reach US$5.4 Billion by 2030 with a CAGR of a 11.9%. The Adult Age Group segment is also set to grow at 7.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.5 Billion in 2024, and China, forecasted to grow at an impressive 13.7% CAGR to reach $2.0 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Combination Vaccines Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Combination Vaccines Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Combination Vaccines Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Combination Vaccines market report include:
- AstraZeneca plc
- Bavarian Nordic A/S
- Bharat Biotech International Ltd.
- Biological E. Limited
- CanSino Biologics Inc.
- CSL Limited (Seqirus)
- Daiichi Sankyo Company, Limited
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc (GSK)
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- Medigen Vaccine Biologics Corporation
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novartis International AG
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Sinovac Biotech Ltd.
- Valneva SE
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AstraZeneca plc
- Bavarian Nordic A/S
- Bharat Biotech International Ltd.
- Biological E. Limited
- CanSino Biologics Inc.
- CSL Limited (Seqirus)
- Daiichi Sankyo Company, Limited
- Emergent BioSolutions Inc.
- GlaxoSmithKline plc (GSK)
- Inovio Pharmaceuticals, Inc.
- Johnson & Johnson
- Medigen Vaccine Biologics Corporation
- Merck & Co., Inc.
- Mitsubishi Tanabe Pharma Corporation
- Novartis International AG
- Pfizer Inc.
- Sanofi Pasteur
- Serum Institute of India Pvt. Ltd.
- Sinovac Biotech Ltd.
- Valneva SE
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 373 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 5.5 Billion |
| Forecasted Market Value ( USD | $ 9.6 Billion |
| Compound Annual Growth Rate | 9.8% |
| Regions Covered | Global |