Global PARP Inhibitor Biomarkers Market - Key Trends & Drivers Summarized
Why Is the PARP Inhibitor Biomarkers Market Gaining Momentum?
The PARP inhibitor biomarkers market is expanding as the role of precision medicine in oncology continues to grow. PARP (poly ADP-ribose polymerase) inhibitors are a class of targeted therapies used in the treatment of cancers such as ovarian, breast, prostate, and pancreatic cancer. The need for predictive biomarkers to identify patients who will respond to PARP inhibitors is driving advancements in companion diagnostics and genomic testing.Additionally, the increasing availability of next-generation sequencing (NGS) and liquid biopsy technologies is enabling more precise biomarker identification, improving treatment selection and patient outcomes. The expansion of clinical trials exploring new applications of PARP inhibitors is further fueling market demand.
How Are Genomic Testing and Companion Diagnostics Transforming PARP Inhibitor Biomarker Identification?
The integration of genomic testing into cancer care is revolutionizing PARP inhibitor biomarker discovery. BRCA1/2 mutation testing, homologous recombination deficiency (HRD) assays, and loss-of-heterozygosity (LOH) analysis are becoming standard tests for predicting PARP inhibitor efficacy. These biomarkers help oncologists tailor treatment plans, improving therapeutic success rates.Companion diagnostics are also playing a crucial role, with regulatory agencies approving biomarker-based tests alongside PARP inhibitor therapies. AI-driven bioinformatics tools are further refining biomarker identification, enhancing accuracy and clinical decision-making.
Is the Expansion of PARP Inhibitor Applications Driving Market Growth?
The growing application of PARP inhibitors beyond ovarian and breast cancer is expanding the market for PARP inhibitor biomarkers. Research is exploring their use in lung, pancreatic, and colorectal cancers, increasing the demand for biomarker testing to guide patient selection.Additionally, the rise of combination therapies involving PARP inhibitors and immune checkpoint inhibitors is driving biomarker innovation. Scientists are developing predictive tests that assess tumor microenvironments and immune response, leading to more personalized treatment strategies.
What’ s Driving the Growth of the PARP Inhibitor Biomarkers Market?
The growth in the PARP inhibitor biomarkers market is driven by the increasing adoption of precision oncology, advancements in genomic testing, and the expansion of PARP inhibitor applications. The rising demand for companion diagnostics and AI-powered biomarker identification is further accelerating market growth.Additionally, regulatory approvals for biomarker-driven therapies, the growing investment in cancer research, and the integration of NGS and liquid biopsy technologies are fueling innovation. As personalized cancer treatment continues to evolve, the demand for PARP inhibitor biomarkers is expected to rise significantly.
Report Scope
The report analyzes the PARP Inhibitor Biomarkers market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product Type (PARP Inhibitor Biomarker Kits, PARP Inhibitor Biomarker Assays); Application (Breast Cancer Application, Ovarian Cancer Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the PARP Inhibitor Biomarker Kits segment, which is expected to reach US$1.0 Billion by 2030 with a CAGR of a 8.8%. The PARP Inhibitor Biomarker Assays segment is also set to grow at 5.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $259.3 Million in 2024, and China, forecasted to grow at an impressive 12.1% CAGR to reach $317.4 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global PARP Inhibitor Biomarkers Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global PARP Inhibitor Biomarkers Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global PARP Inhibitor Biomarkers Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Bosch, Continental AG, DENSO Corporation, Valeo, Aptiv and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this PARP Inhibitor Biomarkers market report include:
- Agilent Technologies, Inc.
- Ambry Genetics
- Amoy Diagnostics Co., Ltd.
- AstraZeneca
- Bio-Rad Laboratories, Inc.
- CENTOGENE N.V.
- Exagen Inc.
- F. Hoffmann-La Roche AG
- Genway Biotech, Inc.
- GlaxoSmithKline (GSK)
- Illumina, Inc.
- Invitae Corporation
- Merck & Co., Inc.
- Myriad Genetics, Inc.
- NeoGenomics Laboratories
- Pfizer Inc.
- QIAGEN
- Siemens Healthcare GmbH
- Svar Life Science AB
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Agilent Technologies, Inc.
- Ambry Genetics
- Amoy Diagnostics Co., Ltd.
- AstraZeneca
- Bio-Rad Laboratories, Inc.
- CENTOGENE N.V.
- Exagen Inc.
- F. Hoffmann-La Roche AG
- Genway Biotech, Inc.
- GlaxoSmithKline (GSK)
- Illumina, Inc.
- Invitae Corporation
- Merck & Co., Inc.
- Myriad Genetics, Inc.
- NeoGenomics Laboratories
- Pfizer Inc.
- QIAGEN
- Siemens Healthcare GmbH
- Svar Life Science AB
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 278 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
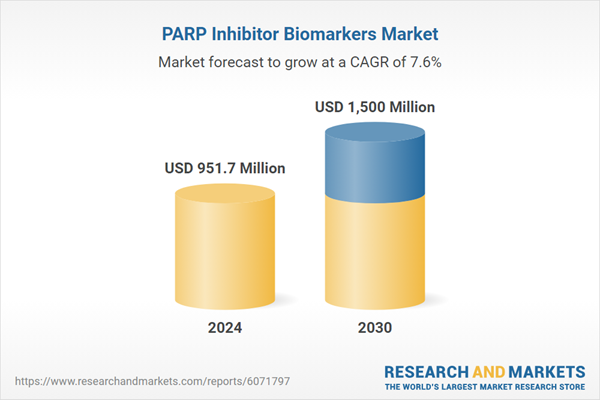
| Estimated Market Value ( USD | $ 951.7 Million |
| Forecasted Market Value ( USD | $ 1500 Million |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |