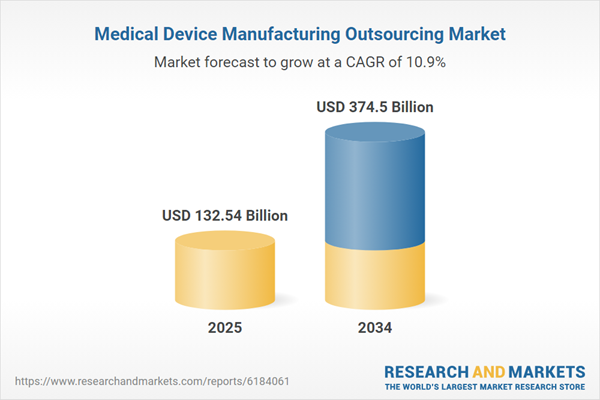
Medical Device Manufacturing Outsourcing Market
The medical device manufacturing outsourcing market comprises contract partners that design, industrialize, and produce devices and components for OEMs across cardiology, orthopedics, minimally invasive surgery, diabetes care, diagnostics/IVD, ophthalmology, wound care, neuromodulation, imaging subassemblies, and hospital consumables. Capabilities span design for manufacturability/assembly (DFM/A), rapid prototyping, precision machining and micro-molding, extrusion and catheter shaft construction, metal implants and instruments, PCBAs and EMS integration, firmware and testing, cleanroom assembly and sterile barrier packaging, along with terminal sterilization and logistics. Current trends prioritize speed-to-market with robust design transfer, digital validation (eDHR/MBR), and vertically integrated “design-to-sterile” offerings. OEMs are dual-sourcing critical platforms, rationalizing supplier bases, and shifting footprints toward nearshore options to improve resilience and lead times. Quality and regulatory depth - ISO 13485, FDA QMSR, EU MDR/IVDR remediation - are mandatory, with increasing scrutiny on cybersecurity, UDI/traceability, and post-market surveillance. Materials and process innovation - bioabsorbables, specialty alloys, antimicrobial surfaces, additive manufacturing for lattices and tooling, high-precision automation - enables miniaturization and consistent throughput. Competitive intensity spans global tier-one CDMOs, large EMS houses entering regulated care, and specialized niche players in catheter/implant manufacturing, as well as packaging and sterilization providers. Differentiation rests on launch reliability, PPAP/validation rigor, OTIF performance, and cost visibility across the product lifecycle. Key challenges include component and resin availability, electronics lead-times, validation and documentation burden, and the need to harmonize ESG reporting (energy, waste, supplier ethics). Partners that combine end-to-end capability, geo-diverse capacity, and lifecycle services - from R&D support to aftermarket and sustaining engineering - are best positioned as OEMs concentrate internal resources on clinical evidence, brand, and market access.Medical Device Manufacturing Outsourcing Market Key Insights
- Design transfer as the make-or-break moment
- From component vendor to platform partner
- Regulatory muscle is a core differentiator
- Electronics and software pull EMS deeper into medtech
- Catheters, MIS, and implantables drive precision demand
- Additive manufacturing moves from prototyping to production
- Geostrategy: nearshoring with redundancy
- Automation and digital factories lift OEE
- Sustainability enters the RFQ
- Commercial models evolve
Medical Device Manufacturing Outsourcing Market Reginal Analysis
North America
Strong demand for regulated device manufacturing with rapid design transfer and robust documentation. Nearshoring to the U.S. and Mexico enhances speed and resilience; electronics and software validation talent are decisive for connected devices. Hospital consolidation and value-analysis committees push cost and quality transparency; ESG and cybersecurity clauses expand in MSAs.Europe
EU MDR/IVDR compliance drives remediation and documentation services alongside manufacturing. Germany, Ireland, and Eastern Europe anchor precision machining, catheter/implant lines, and packaging centers. OEMs emphasize eco-design and supplier LCA disclosures; multilingual regulatory support and stable lead times are critical amid tight labor markets.Asia-Pacific
Scale manufacturing in China, Malaysia, Singapore, and Thailand supports high-volume disposables, EMS, and precision plastics; India grows in implants, instruments, and catheters with strong engineering talent. Regional players invest in cleanrooms, automation, and global certifications to win export programs; supply-chain agility and competitive lead times differentiate.Middle East & Africa
Nascent but growing with government initiatives for local device assembly and sterile packaging. Focus on basic disposables and kits, with select specialty lines co-developed with global CDMOs. Regulatory capacity building and technology transfer partnerships define early opportunities; reliability and training support are key award factors.South & Central America
Brazil and Mexico lead with clusters serving regional demand and nearshore exports. Local content rules and tender preferences encourage partial localization; sterilization and packaging hubs support imports and regional builds. Currency volatility favors suppliers offering inventory buffers, flexible contracts, and strong QA/RA interfaces to maintain continuity.Medical Device Manufacturing Outsourcing Market Segmentation
By Product
- EMS
- Raw Materials
- Finished goods
By Regulatory Classification
- Class II
- Class III
- Class I
Key Market players
Jabil Healthcare, Flex Health Solutions, Sanmina, Celestica, Plexus Corp., Benchmark Electronics, Kimball Electronics, Integer Holdings, Viant Medical, Phillips-Medisize (Molex), TE Connectivity (Medical), SMC Ltd., Tegra Medical, Cretex Medical, Nordson MEDICALMedical Device Manufacturing Outsourcing Market Analytics
The report employs rigorous tools, including Porter’s Five Forces, value chain mapping, and scenario-based modelling, to assess supply-demand dynamics. Cross-sector influences from parent, derived, and substitute markets are evaluated to identify risks and opportunities. Trade and pricing analytics provide an up-to-date view of international flows, including leading exporters, importers, and regional price trends.Macroeconomic indicators, policy frameworks such as carbon pricing and energy security strategies, and evolving consumer behaviour are considered in forecasting scenarios. Recent deal flows, partnerships, and technology innovations are incorporated to assess their impact on future market performance.
Medical Device Manufacturing Outsourcing Market Competitive Intelligence
The competitive landscape is mapped through proprietary frameworks, profiling leading companies with details on business models, product portfolios, financial performance, and strategic initiatives. Key developments such as mergers & acquisitions, technology collaborations, investment inflows, and regional expansions are analyzed for their competitive impact. The report also identifies emerging players and innovative startups contributing to market disruption.Regional insights highlight the most promising investment destinations, regulatory landscapes, and evolving partnerships across energy and industrial corridors.
Countries Covered
- North America - Medical Device Manufacturing Outsourcing market data and outlook to 2034
- United States
- Canada
- Mexico
- Europe - Medical Device Manufacturing Outsourcing market data and outlook to 2034
- Germany
- United Kingdom
- France
- Italy
- Spain
- BeNeLux
- Russia
- Sweden
- Asia-Pacific - Medical Device Manufacturing Outsourcing market data and outlook to 2034
- China
- Japan
- India
- South Korea
- Australia
- Indonesia
- Malaysia
- Vietnam
- Middle East and Africa - Medical Device Manufacturing Outsourcing market data and outlook to 2034
- Saudi Arabia
- South Africa
- Iran
- UAE
- Egypt
- South and Central America - Medical Device Manufacturing Outsourcing market data and outlook to 2034
- Brazil
- Argentina
- Chile
- Peru
Research Methodology
This study combines primary inputs from industry experts across the Medical Device Manufacturing Outsourcing value chain with secondary data from associations, government publications, trade databases, and company disclosures. Proprietary modeling techniques, including data triangulation, statistical correlation, and scenario planning, are applied to deliver reliable market sizing and forecasting.Key Questions Addressed
- What is the current and forecast market size of the Medical Device Manufacturing Outsourcing industry at global, regional, and country levels?
- Which types, applications, and technologies present the highest growth potential?
- How are supply chains adapting to geopolitical and economic shocks?
- What role do policy frameworks, trade flows, and sustainability targets play in shaping demand?
- Who are the leading players, and how are their strategies evolving in the face of global uncertainty?
- Which regional “hotspots” and customer segments will outpace the market, and what go-to-market and partnership models best support entry and expansion?
- Where are the most investable opportunities - across technology roadmaps, sustainability-linked innovation, and M&A - and what is the best segment to invest over the next 3-5 years?
Your Key Takeaways from the Medical Device Manufacturing Outsourcing Market Report
- Global Medical Device Manufacturing Outsourcing market size and growth projections (CAGR), 2024-2034
- Impact of Russia-Ukraine, Israel-Palestine, and Hamas conflicts on Medical Device Manufacturing Outsourcing trade, costs, and supply chains
- Medical Device Manufacturing Outsourcing market size, share, and outlook across 5 regions and 27 countries, 2023-2034
- Medical Device Manufacturing Outsourcing market size, CAGR, and market share of key products, applications, and end-user verticals, 2023-2034
- Short- and long-term Medical Device Manufacturing Outsourcing market trends, drivers, restraints, and opportunities
- Porter’s Five Forces analysis, technological developments, and Medical Device Manufacturing Outsourcing supply chain analysis
- Medical Device Manufacturing Outsourcing trade analysis, Medical Device Manufacturing Outsourcing market price analysis, and Medical Device Manufacturing Outsourcing supply/demand dynamics
- Profiles of 5 leading companies - overview, key strategies, financials, and products
- Latest Medical Device Manufacturing Outsourcing market news and developments
Additional Support
With the purchase of this report, you will receive:- An updated PDF report and an MS Excel data workbook containing all market tables and figures for easy analysis.
- 7-day post-sale analyst support for clarifications and in-scope supplementary data, ensuring the deliverable aligns precisely with your requirements.
- Complimentary report update to incorporate the latest available data and the impact of recent market developments.
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Jabil Healthcare
- Flex Health Solutions
- Sanmina
- Celestica
- Plexus Corp.
- Benchmark Electronics
- Kimball Electronics
- Integer Holdings
- Viant Medical
- Phillips-Medisize (Molex)
- TE Connectivity (Medical)
- SMC Ltd.
- Tegra Medical
- Cretex Medical
- Nordson MEDICAL
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 160 |
| Published | November 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 132.54 Billion |
| Forecasted Market Value ( USD | $ 374.5 Billion |
| Compound Annual Growth Rate | 10.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |