Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Maternal Age and Associated Risk of Chromosomal Abnormalities
The growing maternal age across Europe significantly drives demand in the Non-Invasive Prenatal Testing (NIPT) market. As more women delay childbirth due to career, financial, and lifestyle choices, the likelihood of chromosomal disorders like Down syndrome (trisomy 21), Edwards syndrome (trisomy 18), and Patau syndrome (trisomy 13) increases. According to the Office for National Statistics (ONS), the average age of mothers in England and Wales rose to 30.9 years in 2023.This demographic trend directly correlates with greater use of NIPT, which is non-invasive, highly accurate, and free of the miscarriage risks posed by conventional methods such as amniocentesis or chorionic villus sampling (CVS). NIPT uses maternal blood samples to analyze cell-free fetal DNA, providing a safe and reliable alternative for prenatal screening. In the UK, the National Health Service (NHS) has integrated NIPT into standard prenatal care, improving accessibility. As the average maternal age continues to rise across Europe, demand for accurate, non-invasive screening methods like NIPT is expected to grow substantially, reinforcing its role in modern prenatal care.
Key Market Challenges
High Cost of NIPT
The elevated cost of non-invasive prenatal testing (NIPT) remains a key barrier to widespread adoption across Europe. Advanced technologies such as next-generation sequencing and the need for specialized lab infrastructure contribute to the high per-test expense. In many countries, public healthcare systems do not fully reimburse NIPT, placing the financial burden on patients. This results in limited access for individuals from lower and middle-income groups, restricting overall market penetration.Additionally, pricing inconsistencies and variable insurance coverage create further challenges for both patients and providers. The cost factor also affects healthcare professionals’ willingness to recommend NIPT and poses budget constraints for broader inclusion in public prenatal screening programs. For diagnostic firms, high operational and production expenses constrain market entry and expansion opportunities. Addressing these financial challenges will require expanded insurance support, government funding, and cost-efficient technological advancements to ensure equitable access to NIPT across the region.
Key Market Trends
Integration of NIPT into Routine Prenatal Care
One of the most significant trends in the European NIPT market is its growing incorporation into routine prenatal care. Advancements in genomics and the shift toward personalized medicine are making NIPT a standard first-line screening method. The test’s accuracy in identifying common chromosomal abnormalities - along with its safety and non-invasive nature - has led healthcare providers to recommend it earlier in pregnancy. National healthcare programs in countries like Belgium and the Netherlands now offer government-funded NIPT to all pregnant women, resulting in high adoption rates.For instance, second-line NIPT uptake in regions like Andalucía, Spain has reached over 93% for eligible cases. Integration into care protocols enhances patient awareness and enables informed decision-making during early antenatal visits. Additionally, user-friendly platforms and automated analysis tools are improving operational efficiency for clinicians. This mainstreaming of NIPT is paving the way for broader utilization and supporting the transition to comprehensive, individualized prenatal care across Europe.
Key Market Players
- F. Hoffmann-La Roche Ltd.
- Eurofins LifeCodexx GmbH
- Yourgene Health plc
- Centogene N.V.
- QIAGEN N.V.
- Eluthia GmbH
- Life Genomics AB
- TATAA Biocenter AB
- Genoma SA
- Synlab International GmbH
Report Scope:
In this report, the Europe Non-Invasive Prenatal Testing (NIPT) Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below.Europe Non-Invasive Prenatal Testing (NIPT) Market, By Product:
- Consumables
- Instruments
Europe Non-Invasive Prenatal Testing (NIPT) Market, By Test Type:
- Materni 21
- Harmony
- Panaroma
- Verifi
- NIFTY
- Others
Europe Non-Invasive Prenatal Testing (NIPT) Market, By Application:
- Trisomy
- Microdeletion Syndrome
- Others
Europe Non-Invasive Prenatal Testing (NIPT) Market, By End User:
- Diagnostic Laboratories
- Hospitals
- Others
Europe Non-Invasive Prenatal Testing (NIPT) Market, By Country:
- Germany
- France
- United Kingdom
- Italy
- Spain
- Russia
- Poland
- Bulgaria
- Finland
- Portugal
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Europe Non-Invasive Prenatal Testing (NIPT) Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- F. Hoffmann-La Roche Ltd.
- Eurofins LifeCodexx GmbH
- Yourgene Health plc
- Centogene N.V.
- QIAGEN N.V.
- Eluthia GmbH
- Life Genomics AB
- TATAA Biocenter AB
- Genoma SA
- Synlab International GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 133 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
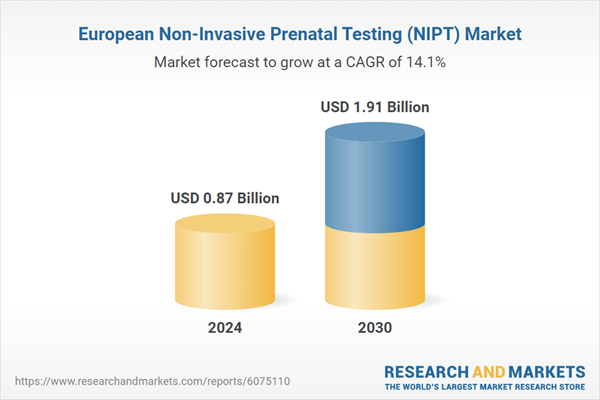
| Estimated Market Value ( USD | $ 0.87 Billion |
| Forecasted Market Value ( USD | $ 1.91 Billion |
| Compound Annual Growth Rate | 14.0% |
| Regions Covered | Europe |
| No. of Companies Mentioned | 10 |