Speak directly to the analyst to clarify any post sales queries you may have.
Diagnostic radiopharmaceuticals are redefining precision imaging by merging molecular biology, isotope logistics, and clinical decision-making at scale
Diagnostic radiopharmaceuticals sit at the intersection of biology, physics, and logistics, enabling clinicians to visualize disease processes in vivo with a level of specificity that conventional imaging cannot always match. From oncology and cardiology to neurology and infection imaging, these agents translate molecular targets into actionable images, supporting earlier detection, therapy selection, and longitudinal monitoring. As health systems push toward precision medicine, diagnostic radiopharmaceuticals increasingly function as decision engines that influence downstream care pathways rather than as stand-alone imaging add-ons.What makes the category uniquely strategic is its dependency on time-sensitive manufacturing and distribution. Many products have short half-lives, which means operational excellence can be as important as clinical performance. As a result, the market’s competitive advantage is often built on integrated capabilities-cyclotron access, generator supply, radiochemistry expertise, quality systems, and reliable last-mile delivery to imaging sites. This integration requirement is also pulling in new stakeholders, including contract development and manufacturing organizations, radiopharmacies, logistics providers, and equipment vendors.
At the same time, innovation in tracers and imaging hardware is changing what “diagnosis” means. Hybrid imaging platforms, improved detector technologies, and quantification workflows are moving the field from primarily qualitative reads to more standardized, measurable biomarkers. Consequently, diagnostic radiopharmaceuticals are becoming more tightly linked to clinical trials, companion diagnostics, and evidence-based reimbursement discussions.
Against this backdrop, stakeholders must navigate regulatory expectations, isotope supply security, evolving provider economics, and competitive pressures across different modalities. The executive summary that follows frames the most important shifts reshaping diagnostic radiopharmaceuticals and outlines how segmentation, regional dynamics, tariff considerations, and company strategies are converging to redefine the industry’s next chapter.
Transformative shifts are reshaping diagnostic radiopharmaceuticals through decentralized supply, quantitative imaging, and theranostic-driven clinical workflows
The landscape is undergoing a transition from traditional, centralized production models toward more distributed and resilient networks. Short-lived isotopes and increasing demand for same-day imaging are motivating investments in regional radiopharmacies and localized production capabilities. This shift is not simply about proximity; it reflects a broader prioritization of supply continuity, predictable scheduling for imaging centers, and reduced risk of missed patient appointments due to transport delays.In parallel, clinical practice is moving from broad, organ-level assessment to target-specific characterization. The rise of theranostics has elevated the role of diagnostic scans as gatekeepers for targeted radionuclide therapy, especially in oncology. That relationship is reinforcing the need for standardized imaging protocols, harmonized quantification, and consistent tracer availability, because therapy decisions are increasingly dependent on diagnostic uptake thresholds and lesion detectability.
Technology is also transforming how radiopharmaceuticals are developed and used. Advances in chelation chemistry, peptide engineering, and antibody fragments are expanding the pipeline beyond legacy tracers. Meanwhile, digital PET, time-of-flight improvements, and software-enabled reconstruction are increasing sensitivity and enabling lower-dose protocols in some settings. As imaging becomes more quantitative, data infrastructure is becoming an innovation layer, with institutions integrating imaging biomarkers into longitudinal patient records and clinical research datasets.
Regulatory and quality expectations are rising in tandem. Authorities are placing greater emphasis on manufacturing controls, sterile production, validation, and traceability across complex supply chains. This is prompting companies to treat quality systems as strategic differentiators rather than compliance necessities. Additionally, workforce dynamics are shifting as the industry competes for radiochemists, qualified persons, medical physicists, and nuclear pharmacists, making talent development and training programs critical enablers.
Finally, collaboration models are evolving. Partnerships between tracer developers, imaging equipment manufacturers, and provider networks are becoming more common, aiming to accelerate adoption and standardize clinical workflows. In effect, the market is moving toward ecosystem competition, where success depends on integrated offerings that combine product, equipment compatibility, service reliability, and evidence generation.
The cumulative impact of prospective US tariffs in 2025 may reshape sourcing, capital spending, and operational economics across radiopharmaceutical supply chains
United States tariff actions anticipated in 2025 are poised to influence diagnostic radiopharmaceuticals in ways that extend beyond direct product imports. While many isotopes and radiopharmaceutical preparations are constrained by half-life and regulatory handling requirements, the broader value chain includes critical inputs-specialty chemicals, shielding materials, single-use sterile components, instrumentation parts, and electronics for imaging and hot-cell environments. Tariff-related cost pressure on these upstream categories can affect both production economics and the pace of capacity expansion.One cumulative effect is a potential reprioritization of supplier strategies. Manufacturers and radiopharmacies may diversify sourcing across multiple geographies, qualify secondary suppliers for key consumables, and build additional inventory buffers for items that are not perishable. However, because many radiopharmaceutical operations depend on validated materials and tightly controlled change management, supplier switching is neither fast nor trivial. This can elevate the value of long-term supplier agreements, dual qualification programs, and proactive regulatory documentation that enables faster substitutions when disruptions occur.
Tariffs may also indirectly shape capital investment cycles. Imaging centers and radiopharmacies that are planning upgrades-such as new cyclotrons, synthesis modules, dose calibrators, and automated dispensing systems-could face higher acquisition and maintenance costs if imported components are affected. In response, organizations may stage investments, renegotiate service contracts, or evaluate refurbished alternatives. Over time, this dynamic can favor vendors with domestic manufacturing footprints or resilient global supply networks.
On the provider side, tariff-driven cost increases can intensify scrutiny of procedure economics. When input costs rise, imaging departments may seek improved scheduling efficiency, reduced dose wastage, and tighter coordination with radiopharmacies to protect margins and maintain patient throughput. This operational pressure tends to accelerate adoption of automation, better forecasting tools, and standardized protocols that reduce variability.
Ultimately, the cumulative impact is likely to be a strategic shift toward supply chain transparency and localization where feasible. Companies that treat tariff volatility as a scenario-planning exercise-rather than a temporary disruption-will be better positioned to maintain service levels, protect quality, and sustain clinical adoption amid changing trade conditions.
Segmentation insights reveal how isotope choice, modality fit, clinical application, end-user economics, and distribution models jointly determine adoption trajectories
Across product type, the market continues to reflect a dual-speed reality in which established SPECT tracers coexist with faster-growing PET agents that enable higher sensitivity and more robust quantification. The product narrative is increasingly defined by clinical purpose: routine functional assessments remain important, yet molecularly targeted imaging is gaining prominence where it can sharpen differential diagnosis, support risk stratification, or guide therapy eligibility.When viewed through radioisotope type, operational considerations strongly influence adoption. Fluorine-18 remains a cornerstone for PET workflows due to its balance of half-life and imaging performance, supporting centralized production with regional distribution. Gallium-68 is associated with generator-based accessibility and on-site preparation flexibility, making it attractive for institutions seeking entry into targeted PET without full cyclotron dependence. Technetium-99m continues to anchor SPECT volume because of entrenched infrastructure and broad clinical familiarity, while iodine-123 retains relevance for specific indications where its imaging characteristics and clinical conventions remain valuable.
Segmentation by application shows oncology as the primary driver of innovation intensity, in part because targeted imaging is increasingly linked to therapeutic decision-making and clinical trial endpoints. Cardiology remains anchored by high-throughput workflows and standardized protocols, with demand influenced by hospital efficiency initiatives and the availability of alternative modalities. Neurology continues to expand in relevance as health systems seek better tools for evaluating neurodegenerative disease, seizure disorders, and movement conditions, while inflammation and infection imaging persists as a clinically important niche where specificity can materially change management.
Modality segmentation highlights how PET and SPECT are evolving in complementary ways. PET adoption benefits from improvements in detector technology, quantitative workflows, and tracer diversity, while SPECT retains a broad installed base and remains integral to cost-conscious imaging strategies in many systems. Importantly, the modality choice is rarely isolated; it is shaped by local radiopharmacy capabilities, scanner availability, physician familiarity, and the institution’s ability to operationalize scheduling around isotope half-life.
End-user segmentation underscores differing priorities across hospitals, diagnostic imaging centers, academic and research institutes, and radiopharmacies. Hospitals emphasize integrated care pathways and predictable supply for inpatient and outpatient scheduling. Imaging centers prioritize throughput, payer alignment, and reliable delivery windows. Academic and research institutes push tracer innovation and protocol refinement, often acting as adoption catalysts. Radiopharmacies-whether hospital-based or commercial-serve as operational linchpins, with performance measured by on-time delivery, quality compliance, and dose management.
Finally, distribution channel dynamics reflect the importance of proximity and service reliability. Direct supply models can support tighter coordination and standardization for larger networks, while third-party distribution and radiopharmacy partnerships expand access for smaller sites. The strongest strategies increasingly align distribution design with clinical demand patterns, ensuring that segmentation choices translate into measurable operational outcomes.
Regional insights show how infrastructure maturity, isotope access, regulatory environments, and provider economics shape adoption across global healthcare systems
In the Americas, mature imaging infrastructure and a strong base of nuclear medicine expertise support continued uptake of both established and emerging tracers. The region’s competitive dynamics often hinge on radiopharmacy coverage, payer alignment, and the ability to translate clinical evidence into routine protocols. As health systems consolidate, procurement and formulary decisions are increasingly centralized, favoring suppliers that can offer reliable service levels across multi-site networks.In Europe, the market reflects a balance between innovation hubs and cost-containment realities. Regulatory consistency goals and cross-border supply considerations elevate the importance of harmonized quality systems and logistics planning. Many countries maintain strong academic and public health ecosystems that support tracer development and clinical validation, while procurement frameworks can place emphasis on standardized pathways and demonstrated clinical utility.
The Middle East is seeing targeted investments in advanced healthcare infrastructure, including imaging centers of excellence and specialized oncology programs. Adoption often depends on the ability to build sustainable radiopharmacy operations, secure isotope supply, and develop trained workforces that can maintain quality compliance. In markets that are expanding capacity, partnerships with experienced operators can accelerate capability-building and reduce ramp-up risk.
Africa presents a highly heterogeneous picture, where leading urban centers are strengthening nuclear medicine services while broader access remains constrained by infrastructure, workforce availability, and supply chain reach. In this context, scalable service models, training programs, and pragmatic equipment and maintenance strategies can be as important as the tracer portfolio itself.
In Asia-Pacific, growth is shaped by expanding healthcare access, increasing cancer incidence, and investments in modern imaging equipment. Large countries are building domestic manufacturing capabilities and strengthening regulatory pathways, which can improve supply reliability over time. The region also features a wide range of adoption maturity, from highly advanced urban networks pursuing quantitative PET workflows to emerging markets focused on establishing consistent SPECT services.
Across regions, a consistent theme is that clinical demand alone does not guarantee adoption. The ability to deliver doses reliably, maintain compliant production, and integrate imaging results into care pathways is what converts interest into sustained utilization. Regional strategies that align service design, training, and evidence generation with local healthcare structures tend to outperform one-size-fits-all approaches.
Company insights highlight how leaders win through tracer innovation, resilient radiopharmacy networks, compliance excellence, and workflow-enabling partnerships
Company strategies in diagnostic radiopharmaceuticals increasingly differentiate along three axes: tracer innovation, manufacturing and distribution resilience, and clinical adoption enablement. Leading participants are investing in pipelines that target clinically meaningful questions-such as improved lesion detectability, higher specificity, and clearer ties to therapy selection-while also building the operational backbone to supply products reliably at scale.Large integrated players continue to leverage end-to-end capabilities that connect isotope procurement, manufacturing, and distribution with established relationships across imaging centers. Their scale can support broader geographic coverage, standardized quality systems, and coordinated education programs for clinicians. At the same time, specialized developers are advancing differentiated tracers and partnering with commercial radiopharmacies or larger manufacturers to solve the last-mile challenge.
Radiopharmacy networks and contract manufacturing organizations are becoming more strategically visible. As tracer portfolios diversify, health systems often prefer partners that can manage multiple products, maintain predictable delivery windows, and provide documentation support for compliance audits. This elevates the role of service reliability as a competitive advantage, especially where imaging schedules are sensitive to delays.
Equipment and technology providers are also influencing market direction. Improvements in PET and SPECT systems, synthesis automation, dispensing technologies, and dose management software can reduce variability and support wider adoption by easing operational burden. The most compelling offerings increasingly pair hardware with workflow integration, training, and service agreements that address uptime and regulatory documentation needs.
Finally, collaboration is reshaping competitive boundaries. Co-development agreements, site-of-care partnerships, and alliances linking diagnostics to therapeutic pathways are expanding. Companies that can align clinical evidence generation, provider training, and consistent supply are best positioned to translate scientific innovation into routine clinical use.
Actionable recommendations focus on supply resilience, protocol standardization, workforce readiness, and partnerships that convert innovation into routine care
Industry leaders can strengthen their position by treating supply reliability as a core product attribute. This means mapping critical inputs, qualifying secondary suppliers, and building change-control playbooks that allow material substitutions without compromising compliance. In addition, organizations should conduct scenario planning for trade and logistics volatility, focusing on consumables, equipment parts, and cold-chain or shielded transport dependencies.To accelerate clinical adoption, leaders should invest in evidence packages that translate into practical protocols. Education that is paired with clear patient selection guidance, standardized acquisition parameters, and interpretation frameworks reduces variability and builds physician confidence. Where possible, aligning diagnostic imaging with therapy decision pathways can deepen clinical relevance and support sustained utilization.
Operationally, radiopharmacies and imaging centers can capture value by reducing dose wastage and improving scheduling precision. Forecasting tools, automation in dispensing and labeling, and tighter coordination between ordering and delivery windows can protect economics while improving patient experience. For multi-site provider networks, central governance of protocols and vendor management can also reduce fragmentation.
From a partnership perspective, leaders should pursue collaborations that solve adoption bottlenecks. Agreements that bundle tracer supply with equipment compatibility support, maintenance responsiveness, and on-site training tend to reduce friction for providers. In parallel, engaging early with regulators on manufacturing changes and validation plans can shorten timelines when network expansion or supplier transitions are required.
Finally, talent strategy deserves executive attention. Building pipelines for radiochemists, nuclear pharmacists, technologists, and medical physicists-through structured training, mentorship, and academic partnerships-can become a durable advantage. In a market where clinical demand can outpace operational capacity, workforce readiness is often the limiting factor that determines whether growth opportunities can be realized.
Methodology integrates rigorous secondary analysis with expert primary validation to reflect real-world radiopharmacy operations and clinical adoption drivers
The research methodology integrates primary and secondary approaches designed to capture both the scientific realities of radiopharmaceutical development and the operational constraints of manufacturing and distribution. Secondary research draws on publicly available materials such as regulatory documentation, peer-reviewed literature, conference proceedings, company reports, patent activity, and procurement and tender frameworks where accessible. This stage establishes a structured understanding of technology pathways, clinical use patterns, and the broader ecosystem of stakeholders.Primary research complements this foundation through interviews and consultations with industry participants across the value chain. Perspectives from manufacturers, radiopharmacies, imaging site operators, clinicians, and technical experts are used to validate workflow assumptions, identify operational bottlenecks, and clarify adoption drivers. Insights are triangulated to reduce bias and to ensure that conclusions reflect real-world constraints such as scheduling, compliance, and isotope handling requirements.
Analytical work emphasizes pattern recognition across segmentation and geography. The approach evaluates how tracer characteristics, isotope half-life, modality compatibility, and end-user economics interact to influence adoption, and it examines how regional infrastructure and policy environments shape feasibility. Where conflicting inputs emerge, the methodology prioritizes evidence consistency, cross-validation among multiple stakeholders, and alignment with established regulatory and clinical practice norms.
Quality control is maintained through iterative review, consistency checks, and terminology standardization to ensure clarity for decision-makers. The result is a decision-oriented synthesis intended to support strategic planning, partnership evaluation, and operational readiness in a rapidly evolving diagnostic radiopharmaceutical environment.
Conclusion synthesizes innovation, operational constraints, and regional realities shaping diagnostic radiopharmaceuticals into a clear strategic outlook
Diagnostic radiopharmaceuticals are moving from a specialized imaging subset to a strategic pillar of precision care, with growing influence on therapy decisions and clinical trial design. This evolution is being propelled by targeted tracer innovation, improved quantitative imaging capabilities, and the maturation of theranostic workflows that rely on diagnostics for patient selection and monitoring.Yet the market’s trajectory is shaped as much by execution as by science. Half-life constraints, compliant manufacturing requirements, workforce limitations, and distribution reliability determine whether innovations reach routine practice. As trade and cost pressures-including tariff-related risks-introduce additional complexity, resilient supply chain design and disciplined operational management become central to competitive success.
Segmentation patterns underscore that adoption is not uniform; it depends on isotope accessibility, modality fit, clinical application priorities, and the economics and capabilities of end users. Regional differences further reinforce that infrastructure maturity and regulatory context can accelerate or slow uptake.
Organizations that combine differentiated tracers with dependable supply, workflow integration, and evidence-driven provider engagement will be best positioned to shape the next phase of diagnostic radiopharmaceuticals. In a field where minutes matter and quality is non-negotiable, strategic clarity paired with operational excellence is what ultimately converts innovation into patient impact.
Table of Contents
7. Cumulative Impact of Artificial Intelligence 2025
17. China Diagnostic Radiopharmaceuticals Market
Companies Mentioned
The key companies profiled in this Diagnostic Radiopharmaceuticals market report include:- Actinium Pharmaceuticals Inc
- Advanced Accelerator Applications SA
- Bayer AG
- Bracco Imaging S.p.A.
- BWXT Medical Ltd
- Cardinal Health
- China Isotope & Radiation Corporation
- Clarity Pharmaceuticals Pty Ltd
- Curium Pharma
- Eckert & Ziegler AG
- Eli Lilly and Company
- GE Healthcare
- Imagen Clinical Ltd
- Isologic Innovative Radiopharmaceuticals Ltd
- ITM Isotope Technologies Munich SE
- Jubilant Pharmova Limited
- Lantheus Holdings Inc
- Nordion Inc
- NorthStar Medical Radioisotopes LLC
- Novartis AG
- Oncoinvent AS
- RadioMedix Inc
- Radiopharm Theranostics Ltd
- Siemens Healthineers
- Telix Pharmaceuticals Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
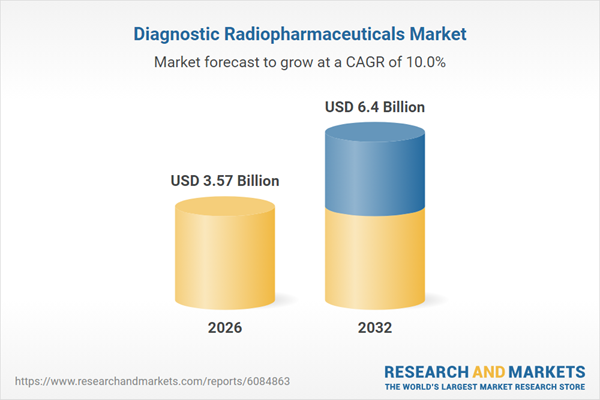
| Forecast Period | 2026 - 2032 |
| Estimated Market Value ( USD | $ 3.57 Billion |
| Forecasted Market Value ( USD | $ 6.4 Billion |
| Compound Annual Growth Rate | 10.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |