Global 'Viral Vectors for Non-Human Primates' Market - Key Trends & Drivers Summarized
Why Are Viral Vectors Gaining Traction in Primate-Based Biomedical Research?
The increasing use of non-human primates (NHPs) in advanced biomedical and neurological research is accelerating the demand for highly efficient viral vectors. These vectors are indispensable tools for delivering genetic material into host cells, enabling researchers to manipulate gene expression with precision. In the context of NHPs, viral vectors are used for studying complex brain functions, neurodegenerative diseases, and cognitive behaviors that closely mimic those in humans. The species similarity of NHPs to humans in terms of neuroanatomy and physiology makes them ideal models for translational research. Adeno-associated viruses (AAVs), lentiviruses, and herpes simplex viruses are among the most commonly used vectors due to their stable expression, high infectivity, and reduced immunogenic responses. The recent surge in interest for optogenetics and gene therapy approaches further cements the role of viral vectors as essential instruments in preclinical studies involving NHPs. The precision required in targeting specific brain regions or genetic sequences in these models has prompted a shift toward tailor-made vectors with neuron-specific promoters and tissue tropism. Consequently, academic institutions, pharmaceutical companies, and biotech firms are investing heavily in viral vector development tailored to primate research.How Are Regulatory Shifts and Ethical Standards Influencing the Landscape?
As viral vector applications in non-human primates expand, global regulatory frameworks are evolving to ensure both scientific integrity and ethical compliance. Regulatory bodies such as the NIH, EMA, and FDA have introduced stringent guidelines that govern the use of NHPs and viral vectors in laboratory settings. These include detailed protocols for biosafety, vector containment, and animal welfare. Ethical considerations are also playing a significant role in shaping research design, pushing stakeholders toward minimal invasiveness and refined vector delivery systems. The 3Rs principle - Replacement, Reduction, and Refinement - is influencing the selection of viral vectors that offer higher transduction efficiency with lower doses, reducing the overall burden on animal subjects. Concurrently, cross-disciplinary collaborations between neuroscientists, virologists, and bioengineers are catalyzing innovations in vector design that are both more effective and ethically sound. The establishment of primate-specific viral vector repositories and open-access platforms is also promoting standardized practices globally. Additionally, increased transparency in data reporting and ethical review processes has begun influencing the choices researchers make when selecting viral vectors for NHP studies, indicating a shift toward greater accountability and sustainability in the field.What Technological Breakthroughs Are Pushing This Field Forward?
Technological innovations are dramatically redefining what is possible in the use of viral vectors for non-human primate research. Advanced vector engineering techniques, such as CRISPR-based genome editing and single-cell sequencing, are enabling more targeted and efficient genetic manipulations. Customizable AAV capsids designed through directed evolution now allow for selective targeting of specific neuronal populations, even within deep brain structures. High-throughput screening technologies have accelerated the discovery of novel vector serotypes with enhanced specificity and minimized immune responses. Simultaneously, improvements in vector manufacturing processes - such as scalable production in suspension cultures and purification via chromatography - are making it feasible to produce clinical-grade vectors suitable for large-scale studies. Moreover, innovations in delivery methods, including stereotactic injection, focused ultrasound-mediated delivery, and minimally invasive catheter-based systems, have significantly improved the precision of vector deployment in primates. AI-driven modeling tools are also being employed to predict vector behavior, optimize dosages, and assess off-target effects. These advances are not only expanding the scope of basic and applied research but are also paving the way for translational studies that could bridge the gap between animal models and human clinical trials.What's Powering the Surge in the Viral Vectors for Non-Human Primates Market?
The growth in the viral vectors for non-human primates market is driven by several factors closely tied to evolving research paradigms, technological advancements, and end-user demands. A primary driver is the increasing reliance on NHPs in neurodegenerative disease modeling, where traditional rodent models fall short. This has created a strong demand for precision tools like viral vectors that can facilitate functional studies of complex brain networks. The expansion of gene therapy pipelines by pharmaceutical and biotech companies is another major catalyst, as these firms require robust preclinical validation in primate models before progressing to human trials. Academic and government research institutions are also ramping up funding for neuroscience and behavioral research that necessitates the use of viral vectors in NHPs. There is a rising emphasis on personalized medicine and biomarker discovery, which further fuels the need for advanced in vivo models enabled by viral vectors. Additionally, the trend toward open science and data sharing has led to collaborative consortia that focus on creating standardized, high-performance vectors for NHP use. Lastly, advancements in primate-specific immunosuppressive strategies have minimized vector rejection, making these tools more viable and reliable in long-term studies. Together, these factors are converging to push the global market into a phase of rapid expansion and diversification.Report Scope
The report analyzes the Viral Vectors for Non-Human Primates market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Vector Type (Adenoviral Vectors, Adeno-Associated Vectors, Retroviral Vectors, Lentiviral Vectors, Other Vector Types); Therapeutic Area (Genetic Disorders, Infectious Diseases, Oncological Disorders, Other Therapeutic Areas); Application (Gene Therapy, Vaccine Research); End-Use (Pharma & Biotech Companies, Academic & Research Institutes).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Adenoviral Vectors segment, which is expected to reach US$100.9 Million by 2030 with a CAGR of a 11.6%. The Adeno-Associated Vectors segment is also set to grow at 7.4% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $42.6 Million in 2024, and China, forecasted to grow at an impressive 13% CAGR to reach $54.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Viral Vectors for Non-Human Primates Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Viral Vectors for Non-Human Primates Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Viral Vectors for Non-Human Primates Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Agrex.ai, AllGoVision Technologies, AvidBeam, Axis Communications AB, Avigilon Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Viral Vectors for Non-Human Primates market report include:
- 4D Molecular Therapeutics
- Andelyn Biosciences
- Barinthus Biotherapeutics
- Beacon Therapeutics
- Biovian Oy
- Catalent Pharma Solutions
- Charles River Laboratories
- CRISPR Therapeutics
- FinVector Oy
- Fujifilm Diosynth Biotechnologies
- Genezen
- Lonza Group AG
- Merck KGaA
- Neuracle Genetics Inc.
- Oxford Biomedica
- REGENXBIO Inc.
- Rocket Pharmaceuticals, Inc.
- SIRION Biotech GmbH
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- 4D Molecular Therapeutics
- Andelyn Biosciences
- Barinthus Biotherapeutics
- Beacon Therapeutics
- Biovian Oy
- Catalent Pharma Solutions
- Charles River Laboratories
- CRISPR Therapeutics
- FinVector Oy
- Fujifilm Diosynth Biotechnologies
- Genezen
- Lonza Group AG
- Merck KGaA
- Neuracle Genetics Inc.
- Oxford Biomedica
- REGENXBIO Inc.
- Rocket Pharmaceuticals, Inc.
- SIRION Biotech GmbH
- Takara Bio Inc.
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 473 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
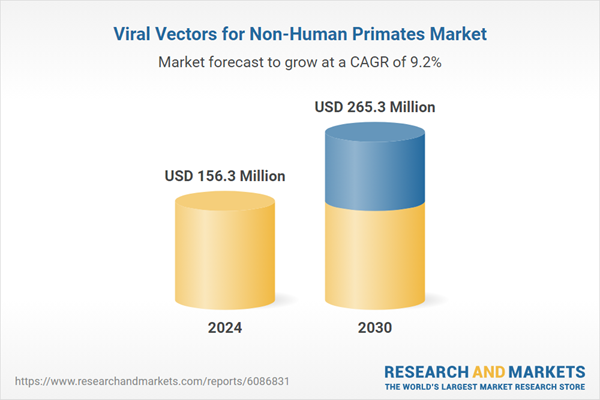
| Estimated Market Value ( USD | $ 156.3 Million |
| Forecasted Market Value ( USD | $ 265.3 Million |
| Compound Annual Growth Rate | 9.2% |
| Regions Covered | Global |