Global m-RNA Synthesis Services Market - Key Trends & Drivers Summarized
Why Are m-RNA Synthesis Services Becoming a Cornerstone in Next-Generation Therapeutics and Vaccine Platforms?
Messenger RNA (mRNA) synthesis services are rapidly evolving into a foundational pillar of the biopharmaceutical ecosystem, particularly in the wake of global attention on mRNA-based COVID-19 vaccines. mRNA functions as the transient intermediary between DNA and protein synthesis, making it a versatile vehicle for encoding disease-specific proteins in therapeutic applications. The demand for high-purity, GMP-grade synthetic mRNA is growing across vaccine development, personalized cancer immunotherapy, protein replacement therapies, and rare disease treatments.Unlike traditional protein or viral vector-based therapeutics, mRNA-based modalities offer rapid, scalable, and cell-free manufacturing advantages. Their ability to elicit targeted immune responses without the risk of genome integration has opened up new possibilities in infectious disease prevention and oncology. Consequently, drug developers - especially biotech startups and academic consortia - are increasingly outsourcing mRNA synthesis to specialized contract development and manufacturing organizations (CDMOs) that provide optimized transcription protocols, capping technologies, and downstream purification expertise.
How Are Technology Platforms and Process Innovations Accelerating the Evolution of m-RNA Synthesis Services?
The technological landscape of mRNA synthesis is advancing rapidly through innovations in in vitro transcription (IVT) chemistry, enzymatic capping, and sequence optimization. Synthetic DNA templates are transcribed using phage RNA polymerases (e.g., T7, SP6), with improvements in template engineering enhancing stability, translational efficiency, and immune evasion. Modified nucleosides like pseudouridine and 5-methylcytidine are being incorporated to reduce innate immune responses and improve mRNA longevity post-administration.Cap analogs (such as CleanCap and ARCA) and poly(A) tail length optimization are now routinely integrated into synthesis workflows, improving ribosome recruitment and translation fidelity. CDMOs are offering end-to-end services - including plasmid linearization, IVT reaction, DNase treatment, chromatographic purification, and sterile fill-finish - under GMP or research-use conditions. The integration of automation, single-use bioreactors, and AI-based quality analytics is reducing cycle times, increasing batch consistency, and ensuring compliance with regulatory expectations for nucleic acid therapeutics.
Which Therapeutic Areas and Developer Segments Are Driving Demand for m-RNA Synthesis Services Globally?
Infectious diseases remain the most prominent therapeutic application, particularly with the success of mRNA vaccines for COVID-19 by Moderna and Pfizer-BioNTech. However, oncology is fast emerging as the next frontier, with mRNA being used to encode tumor-specific neoantigens for individualized immunotherapy. Research is also expanding into cardiovascular diseases, autoimmune conditions, metabolic disorders, and genetic deficiencies - where mRNA can be programmed to express functional proteins in vivo, offering transient but potent therapeutic effects.Academic labs, small biotech firms, and mRNA-focused startups are the most active clients of synthesis service providers due to limited in-house GMP capabilities and the high cost of cleanroom biomanufacturing infrastructure. In parallel, large pharmaceutical companies are increasingly partnering with CDMOs to expand their mRNA pipelines, leveraging external expertise in nucleic acid synthesis, scale-up, and formulation. Government agencies and public-private partnerships are also fueling demand through vaccine stockpile development and pandemic preparedness initiatives.
What Is Driving Long-Term Growth and Competitive Differentiation in the m-RNA Synthesis Services Market?
The growth in the mRNA synthesis services market is driven by the expanding application scope of mRNA-based therapeutics, growing VC investment in mRNA startups, and increasing acceptance of nucleic acid-based modalities by regulatory agencies. Global regulatory frameworks are being adapted to fast-track mRNA candidates under breakthrough therapy and accelerated approval pathways, encouraging development and outsourcing activity. The rise of personalized medicine and neoantigen-specific cancer therapies is fostering a surge in demand for small-batch, customized mRNA synthesis services.To differentiate in a competitive market, service providers are focusing on platform scalability, IP-protected cap analogs, GMP compliance, and integration with lipid nanoparticle (LNP) formulation services. Some are vertically integrating into DNA template manufacturing and RNA stabilization technologies to offer full-cycle RNA drug development support. As mRNA transitions from emergency vaccine use to mainstream therapeutic applications, robust, reliable, and regulatory-compliant synthesis services will be essential to accelerating innovation and ensuring commercial success in this high-growth segment.
Report Scope
The report analyzes the m-RNA Synthesis Services market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Therapeutic Development, Vaccine Production, Drug Discovery, Other Applications); Scale of Operation (Research, Commercial); End-User (Biopharmaceutical Companies, Contract Research Organizations, Other End-Users).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Therapeutic Development Application segment, which is expected to reach US$4.4 Billion by 2030 with a CAGR of a 6.6%. The Vaccine Production Application segment is also set to grow at 4.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.9 Billion in 2024, and China, forecasted to grow at an impressive 5.1% CAGR to reach $1.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global m-RNA Synthesis Services Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global m-RNA Synthesis Services Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global m-RNA Synthesis Services Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Advanced Arm Dynamics, Aether Biomedical, Bionic Prosthetics & Orthotics Group, BionX Medical Technologies, Blatchford Limited and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this m-RNA Synthesis Services market report include:
- AGC Biologics
- Aldevron
- APExBIO
- Aurigene Pharmaceutical Services
- Biomay
- BioNTech SE
- CELLSCRIPT
- Creative Biogene
- CureVac N.V.
- Eurofins Genomics
- GENEWIZ (Azenta Life Sciences)
- GenScript
- Integrated DNA Technologies
- Merck KGaA
- Moderna, Inc.
- New England Biolabs
- Samsung Biologics
- ST Pharm
- Thermo Fisher Scientific
- TriLink BioTechnologies
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AGC Biologics
- Aldevron
- APExBIO
- Aurigene Pharmaceutical Services
- Biomay
- BioNTech SE
- CELLSCRIPT
- Creative Biogene
- CureVac N.V.
- Eurofins Genomics
- GENEWIZ (Azenta Life Sciences)
- GenScript
- Integrated DNA Technologies
- Merck KGaA
- Moderna, Inc.
- New England Biolabs
- Samsung Biologics
- ST Pharm
- Thermo Fisher Scientific
- TriLink BioTechnologies
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 227 |
| Published | January 2026 |
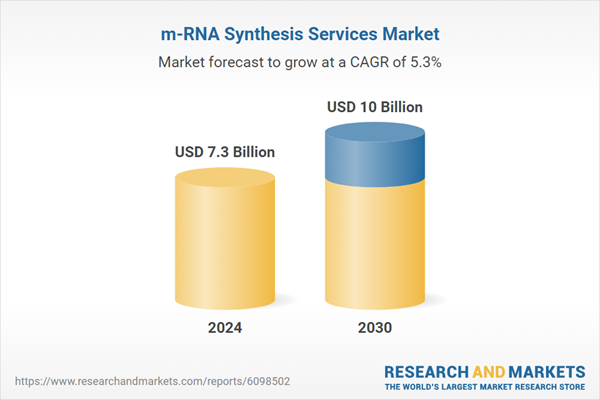
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 7.3 Billion |
| Forecasted Market Value ( USD | $ 10 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |