Global Peptide API Market - Key Trends & Drivers Summarized
Why Are Peptide APIs Gaining Strategic Importance in Drug Discovery and Therapeutic Innovation?
Peptide Active Pharmaceutical Ingredients (APIs) have emerged as one of the most promising segments in modern drug development, offering high specificity, potency, and safety across a wide range of therapeutic indications. Derived from short chains of amino acids, peptide APIs are biologically active compounds that mimic endogenous molecules and interact with specific targets such as receptors, enzymes, and ion channels. Their unique mode of action, favorable pharmacokinetics, and low toxicity make them highly valuable in treating cancer, metabolic disorders, cardiovascular diseases, and rare genetic conditions.The increasing success of peptide-based drugs such as liraglutide, semaglutide, buserelin, and leuprolide has demonstrated the clinical viability of peptides, propelling pharmaceutical firms to invest in specialized R&D and scalable manufacturing platforms. With the shift toward precision medicine and biologics, peptides are bridging the gap between small molecules and large proteins, offering opportunities for novel therapeutic modalities, including cell-penetrating peptides, peptide-drug conjugates, and multifunctional peptides. The expanding pipeline of peptide therapeutics across clinical phases underscores the rising demand for high-purity, cost-efficient peptide APIs.
How Are Advances in Synthesis Techniques and Process Optimization Enhancing API Manufacturing Capabilities?
The evolution of solid-phase peptide synthesis (SPPS), liquid-phase synthesis, and hybrid techniques has significantly improved the scalability, purity, and throughput of peptide API production. Automated peptide synthesizers, improved resins, and optimized coupling reagents now enable efficient synthesis of long, complex, and cyclic peptides with reduced reaction time and waste generation. Additionally, innovations in purification technologies such as preparative HPLC, ion-exchange chromatography, and ultrafiltration have enhanced yield and impurity control.Manufacturers are investing in continuous manufacturing platforms and high-throughput parallel synthesis systems to reduce batch variability and enable rapid scale-up for clinical and commercial supply. Moreover, regulatory expectations for ICH Q11 compliance, process validation, and impurity profiling are driving the adoption of advanced analytics and quality-by-design (QbD) frameworks. Peptide modification strategies, including lipidation, PEGylation, and cyclization, are being integrated at the API stage to enhance bioavailability and stability. These advancements are improving cost-efficiency, regulatory compliance, and therapeutic performance across the peptide value chain.
Which Therapeutic Areas and Regional Markets Are Fueling Global Demand for Peptide APIs?
Metabolic diseases - especially Type 2 diabetes and obesity - remain the primary therapeutic drivers for peptide APIs, given the commercial success of GLP-1 analogs. Oncology, endocrinology, urology, and reproductive health are also major therapeutic domains utilizing peptides for hormone regulation, tumor suppression, and targeted therapy delivery. Peptides are increasingly being used in orphan drug development, vaccine formulations, and infectious disease management, including antimicrobial and antiviral applications.North America and Europe dominate peptide API consumption due to strong biopharmaceutical pipelines, advanced manufacturing capabilities, and regulatory maturity. The U.S. leads in innovation, clinical trials, and commercialization, while Germany, Switzerland, and Belgium are major manufacturing hubs. Asia-Pacific - particularly India, China, South Korea, and Japan - is rapidly expanding its footprint as a cost-efficient supplier of peptide APIs and contract manufacturing services (CDMOs). Regional players are scaling up to meet the needs of both branded innovators and biosimilar developers, creating a globally integrated and competitive market landscape.
What Is Driving Long-Term Growth and Strategic Transformation in the Peptide API Market?
The growth in the peptide API market is driven by rising clinical success rates of peptide drugs, strong R&D investment in biologics, and increasing preference for targeted therapies. As pharmaceutical pipelines diversify and regulatory agencies encourage orphan drug development, peptide APIs are gaining ground as scalable, versatile therapeutic platforms. The global expansion of contract development and manufacturing services (CDMO) is also driving API outsourcing, with specialized peptide facilities in high demand for GMP-compliant, cost-competitive production.Strategically, companies are adopting vertical integration, partnering with biotech firms for co-development, and investing in dual manufacturing sites for supply resilience. The convergence of peptide synthesis with computational biology, AI-based drug design, and nanotechnology is opening new frontiers in personalized medicine and advanced delivery systems. As regulatory frameworks continue to evolve to accommodate peptide therapeutics, API manufacturers that offer flexible, end-to-end solutions with robust quality systems are well-positioned to lead this high-growth segment.
Report Scope
The report analyzes the Peptide API market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Drug Class (GLP-1 Receptor Agonist, Insulin, Other Peptide Classes); Administration Route (Oral Route, Parenteral Route); Application (Type 2 Diabetes Mellitus Application, Type 1 Diabetes Application, Obesity Application, Non-alcoholic Fatty Liver Disease Application, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the GLP-1 Receptor Agonist segment, which is expected to reach US$17.6 Billion by 2030 with a CAGR of a 19.9%. The Insulin segment is also set to grow at 21.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3 Billion in 2024, and China, forecasted to grow at an impressive 27.4% CAGR to reach $7.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Peptide API Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Peptide API Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Peptide API Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ABP (Stichting Pensioenfonds ABP), APG Groep NV, ATP (Arbejdsmarkedets Tillægspension), AustralianSuper, Aware Super and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Peptide API market report include:
- Aji Bio-Pharma Services
- AmbioPharm, Inc.
- Anthem Biosciences
- Bachem Holding AG
- Biocon Limited
- Biopeptek Pharmaceuticals
- CordenPharma International
- CPC Scientific Inc.
- Creative Peptides
- GenScript Biotech Corp.
- Hybio Pharmaceutical Co.
- Innovent Biologics
- Lonza Group AG
- Merck KGaA
- Peptide Institute, Inc.
- PeptiDream Inc.
- PeptiStar Inc.
- PolyPeptide Group
- ScinoPharm Taiwan, Ltd.
- Teva Active Pharmaceutical Ingredients
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aji Bio-Pharma Services
- AmbioPharm, Inc.
- Anthem Biosciences
- Bachem Holding AG
- Biocon Limited
- Biopeptek Pharmaceuticals
- CordenPharma International
- CPC Scientific Inc.
- Creative Peptides
- GenScript Biotech Corp.
- Hybio Pharmaceutical Co.
- Innovent Biologics
- Lonza Group AG
- Merck KGaA
- Peptide Institute, Inc.
- PeptiDream Inc.
- PeptiStar Inc.
- PolyPeptide Group
- ScinoPharm Taiwan, Ltd.
- Teva Active Pharmaceutical Ingredients
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 370 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
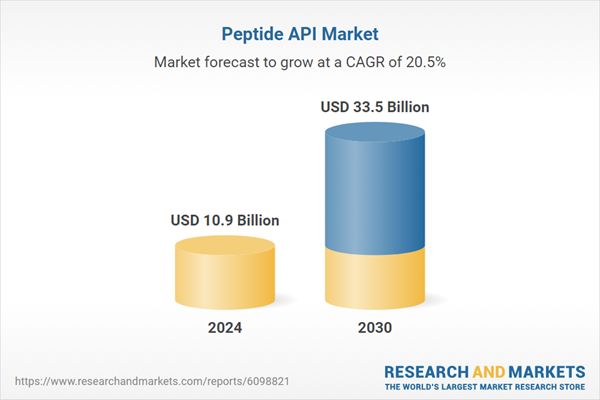
| Estimated Market Value ( USD | $ 10.9 Billion |
| Forecasted Market Value ( USD | $ 33.5 Billion |
| Compound Annual Growth Rate | 20.5% |
| Regions Covered | Global |