Global 'RNAi Drug Delivery' Market - Key Trends & Drivers Summarized
What Makes RNAi Delivery Mechanisms So Crucial in Modern Therapeutics?
RNA interference (RNAi) has revolutionized gene silencing, offering a powerful tool to inhibit disease-causing genes with unmatched precision. However, the crux of translating RNAi therapies from bench to bedside lies in effective delivery systems. Naked siRNA is inherently unstable in the bloodstream, prone to rapid degradation by nucleases and clearance via renal filtration. Additionally, siRNAs are negatively charged and hydrophilic, posing a major hurdle to crossing cellular membranes. This challenge has propelled intense research into designing delivery platforms that not only protect siRNA but also ensure tissue specificity, minimal off-target effects, and efficient cellular uptake. Lipid nanoparticles (LNPs), polymer-based carriers, and conjugation strategies like GalNAc-siRNA represent key modalities in this evolution. Each platform comes with distinct advantages - LNPs are excellent for liver targeting, while polymeric carriers offer versatility and structural tunability. Moreover, extracellular vesicles and aptamer-based carriers are emerging as bio-inspired, less immunogenic alternatives. Without optimized delivery systems, the therapeutic potential of RNAi remains largely untapped, hence making delivery science an indispensable pillar in RNAi-based drug development.Are Innovations in Lipid Nanoparticles & Polymers Reshaping the RNAi Drug Delivery Landscape?
The refinement of lipid-based nanoparticles has taken center stage in enabling clinical success of RNAi drugs, most notably with the approval of patisiran, the first FDA-sanctioned siRNA therapeutic, which uses LNPs to treat hereditary transthyretin-mediated amyloidosis. These nanoparticles encapsulate siRNA, shielding it from enzymatic degradation and facilitating endosomal escape once inside the target cell. Key innovations have involved enhancing ionizable lipids to improve biocompatibility and biodistribution, allowing for reduced dosing frequencies. Parallel to this, polymer-based systems such as polyethyleneimine (PEI), poly(lactic-co-glycolic acid) (PLGA), and dendrimers have been adapted to suit RNAi transport. These offer the advantage of controlled release and reduced systemic toxicity when modified appropriately. Further enhancements include pH-responsive polymers and stimuli-sensitive carriers that release siRNA upon encountering specific intracellular environments. The development of hybrid systems combining both lipid and polymeric components aims to leverage the best features of both. Research is also targeting mucosal and intranasal delivery systems, allowing localized, non-invasive delivery for respiratory or gastrointestinal diseases. Overall, technological innovation in LNPs and polymers is central to expanding the therapeutic index of RNAi therapeutics across a wide range of indications.Can Targeting Specific Organs and Diseases Unlock the Full Potential of RNAi Delivery?
The success of RNAi-based therapies is increasingly dictated by their capacity for organ and cell-type specificity. The liver, owing to its fenestrated endothelium and natural affinity for nanoparticle accumulation, remains the primary target for RNAi drugs today. GalNAc conjugation technology, which exploits the asialoglycoprotein receptor (ASGPR) pathway in hepatocytes, has simplified subcutaneous delivery of siRNA, enabling convenient and targeted therapy for chronic liver diseases. Beyond hepatic applications, researchers are actively exploring delivery to hard-to-reach tissues such as the brain, lungs, and eyes. Blood-brain barrier (BBB) penetration remains a significant hurdle; however, ligand-targeted nanoparticles, focused ultrasound with microbubbles, and exosome-based carriers are showing promise in preclinical models. In pulmonary conditions, aerosolized RNAi formulations are gaining momentum, offering non-invasive and site-specific administration. Similarly, ocular delivery is benefiting from biodegradable implants and hydrogel systems that allow for sustained, localized gene silencing. Cancer remains a compelling but complex target - tumor microenvironment heterogeneity and immune barriers have necessitated the design of smart delivery systems capable of site-specific accumulation, triggered release, and minimal immunogenicity. Tailoring delivery systems for disease-specific physiology is critical to unlocking RNAi's full clinical potential beyond hepatic disorders.Is the Rapid Expansion of the RNAi Market Fuelled by Demand Shifts and Tech Breakthroughs?
The growth in the& global RNAi drug delivery& market is driven by several factors related to technological advancements, evolving end-use patterns, and shifting consumer demands. A primary driver is the exponential rise in chronic and genetic diseases, which has created an urgent need for precision therapeutics - an area where RNAi excels. Increased R&D investments from biopharma giants and startups alike are accelerating clinical pipelines, with several RNAi therapies already progressing through late-stage trials. Moreover, advances in high-throughput screening and bioinformatics tools are enabling rapid identification of novel RNAi targets, shortening development timelines. On the end-user front, the healthcare industry's shift toward personalized medicine is boosting demand for customizable RNAi treatments. Additionally, the preference for minimally invasive administration routes - such as subcutaneous and intranasal - has intensified innovation in delivery systems. The commercial success of initial RNAi drugs has also increased investor confidence, triggering more funding toward RNAi-focused biotech firms. Regulatory agencies are becoming more accommodating as well, offering fast-track approvals and orphan designations for RNAi drugs targeting rare diseases. Furthermore, favorable reimbursement frameworks, especially in North America and Europe, are expanding patient access. The increasing adoption of RNAi in research and diagnostics further propels the market, making it one of the most dynamic segments in modern pharmaceutical innovation.Report Scope
The report analyzes the RNAi Drug Delivery market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Technology (Nanoparticle Drug Delivery, Pulmonary Drug Delivery, Nucleic Acid Drug Delivery, Aptamer Drug Delivery); Application (Infectious Disease, Cardiology, Oncology, Neurology, Ophthalmology, Urology, Metabolic Disorders, Other Applications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Nanoparticle Drug Delivery segment, which is expected to reach US$1.6 Billion by 2030 with a CAGR of a 22.3%. The Pulmonary Drug Delivery segment is also set to grow at 17% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $302.2 Million in 2024, and China, forecasted to grow at an impressive 26.3% CAGR to reach $755.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global RNAi Drug Delivery Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global RNAi Drug Delivery Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global RNAi Drug Delivery Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alibaba Group (T-Head), Allwinner Technology, Andes Technology Corporation, Arteris, Inc., Bluespec, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 39 companies featured in this RNAi Drug Delivery market report include:
- Alnylam Pharmaceuticals
- Aro Biotherapeutics
- Arrowhead Pharmaceuticals
- AstraZeneca
- BioNTech SE
- Dicerna Pharmaceuticals (acquired by Novo Nordisk)
- DTx Pharma (acquired by Novartis)
- GlaxoSmithKline (GSK)
- Ionis Pharmaceuticals
- Moderna, Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Roche Holding AG
- Sanofi
- Sarepta Therapeutics
- Silence Therapeutics
- Sirnaomics
- Switch Therapeutics
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alnylam Pharmaceuticals
- Aro Biotherapeutics
- Arrowhead Pharmaceuticals
- AstraZeneca
- BioNTech SE
- Dicerna Pharmaceuticals (acquired by Novo Nordisk)
- DTx Pharma (acquired by Novartis)
- GlaxoSmithKline (GSK)
- Ionis Pharmaceuticals
- Moderna, Inc.
- Novartis AG
- Novo Nordisk A/S
- Pfizer Inc.
- Regeneron Pharmaceuticals
- Roche Holding AG
- Sanofi
- Sarepta Therapeutics
- Silence Therapeutics
- Sirnaomics
- Switch Therapeutics
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 294 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
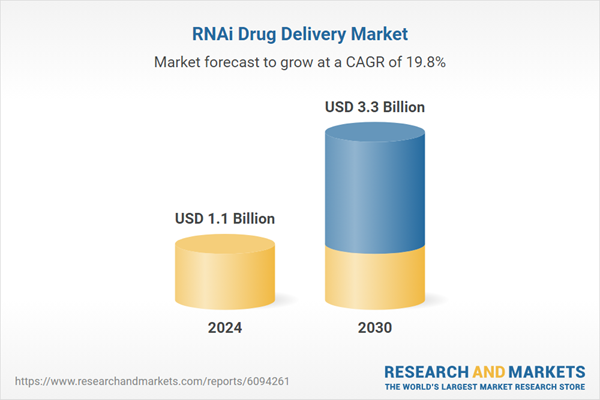
| Estimated Market Value ( USD | $ 1.1 Billion |
| Forecasted Market Value ( USD | $ 3.3 Billion |
| Compound Annual Growth Rate | 19.8% |
| Regions Covered | Global |