Speak directly to the analyst to clarify any post sales queries you may have.
The fecal transplant therapy market is moving rapidly from niche innovation to global clinical adoption, presenting strategic opportunities and operational considerations for healthcare leaders seeking more effective patient outcomes and cost-efficient pathways.
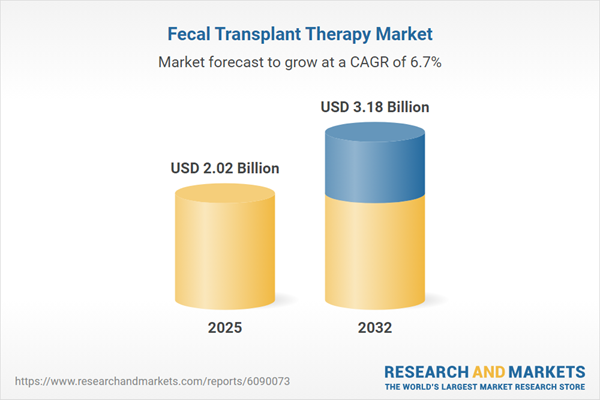
Market Snapshot: Fecal Transplant Therapy Market Size and Growth
The fecal transplant therapy market grew from USD 1.90 billion in 2024 to USD 2.02 billion in 2025. It is expected to continue growing at a CAGR of 6.65%, reaching USD 3.18 billion by 2032. This market maturity is being driven by mounting clinical evidence, evolving regulatory frameworks, and expanding applications across multiple disease areas.
Scope & Segmentation
- Therapy Types: Capsule-based and liquid-based fecal microbiota therapy address distinct needs in procedural settings and patient usability.
- Disease Applications: Targeted conditions include Clostridium difficile infection, autism spectrum disorder, inflammatory bowel disease, irritable bowel syndrome, diabetes mellitus, obesity, and multiple sclerosis.
- Procedural Approaches: Administration methods range from colonoscopy and enema to nasogastric tube and oral capsules to improve both effectiveness and patient experience.
- End Users: The market serves academic and research institutes, ambulatory surgical centers, and hospitals and clinics, each with unique roles in therapy adoption.
- Regional Coverage: Analysis spans Americas, Europe, Middle East & Africa, and Asia-Pacific regions, considering both mature and emerging markets such as the United States, Germany, United Arab Emirates, China, and Brazil.
- Industry Participants: Key companies comprise AOBiome Therapeutics, Assembly Biosciences, BiomX, Evogene, Ferring Pharmaceuticals, Finch Therapeutics, MaaT Pharma, Nestlé Health Science, OpenBiome, Seres Therapeutics, Takeda, Theriva Biologics, Vedanta Biosciences, and Taconic Biosciences.
Key Takeaways: Strategic Insights for Senior Decision-Makers
- Fecal transplant therapy is expanding beyond gastrointestinal diseases, with growing relevance for metabolic, immune, and neuropsychiatric disorders, reflecting emerging clinical needs.
- Standardization of donor screening and manufacturing protocols is driving safer and more scalable product offerings, supported by centralization efforts within stool banks and clinical networks.
- Technological advancements, especially high-throughput sequencing and bioinformatics, have accelerated the development of patient-specific therapies and precision microbiome interventions.
- The ongoing shift toward outpatient and ambulatory delivery, particularly with capsule-based methods, is reducing logistical barriers while increasing patient adherence and satisfaction.
- Integrated care models and multi-sector collaboration among clinical centers, research institutions, and pharmaceutical companies are underpinning therapy innovation and broader adoption.
Tariff Impact: Supply Chain and Cost Pressures (United States, 2025)
Recent tariff adjustments on laboratory equipment, consumables, and cold chain components in the United States are increasing supply chain complexity and procurement costs for therapy developers. Stakeholders are responding with regional manufacturing strategies, consolidated supply agreements, and a drive toward greater supply chain resilience. The evolving trade environment is expected to prompt both short-term challenges and long-term opportunities for operational agility and cost management.
Methodology & Data Sources
This report is based on a robust methodology combining secondary research from peer-reviewed journals, regulatory sources, and industry documentation with primary interviews from clinical, regulatory, and supply chain experts. Analytical models and scenario-based validation ensure that findings provide a reliable market foundation for planning and decision-making.
Why This Report Matters
- Supports strategic investment and expansion planning by offering comprehensive market segmentation aligned with evolving indications and regions.
- Offers clarity on regulatory and tariff shifts, enabling more resilient and cost-effective supply chain decisions for microbiome therapeutics.
- Equips decision-makers with actionable insights on technology trends and end-user adoption, supporting the successful launch and scaling of fecal transplant therapies.
Conclusion
As clinical acceptance broadens and regulatory clarity increases, fecal transplant therapy is set to play an expanded role across global healthcare markets. Senior leaders who act on these insights will be positioned to shape the next phase of innovation, operationalize best practices, and capitalize on emerging opportunities in this evolving therapeutic landscape.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Fecal Transplant Therapy Market report include:- AOBiome Therapeutics, Inc.
- Assembly Biosciences, Inc.
- BiomX Inc.
- Evogene Ltd.
- Ferring Pharmaceuticals
- Finch Therapeutics Group, Inc.
- MaaT Pharma
- Nestlé Health Science
- OpenBiome
- Seres Therapeutics, Inc.
- Takeda Pharmaceutical Company Limited
- Theriva Biologics, Inc.
- Vedanta Biosciences, Inc.
- Taconic Biosciences, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 184 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 2.02 Billion |
| Forecasted Market Value ( USD | $ 3.18 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 15 |