Speak directly to the analyst to clarify any post sales queries you may have.
The clinical trial services market is rapidly evolving as new technologies, regulatory changes, and shifting stakeholder priorities drive industry transformation. Senior leaders in life sciences and healthcare are increasingly leveraging robust clinical trial service solutions to optimize drug development, ensure compliance, and deliver patient-focused innovations.
Market Snapshot: Current Growth and Dynamics
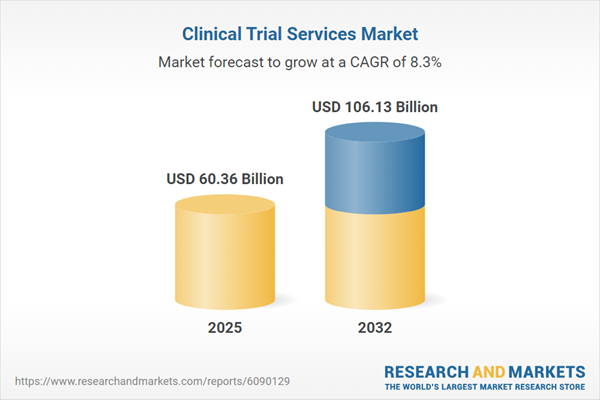
The clinical trial services market grew from USD 55.93 billion in 2024 to USD 60.36 billion in 2025, maintaining strong momentum with a projected CAGR of 8.33%. The sector is anticipated to reach USD 106.13 billion by 2032, indicating sustained demand for efficient, high-quality clinical research solutions. Market growth reflects the broadening of trial complexity, adoption of digital solutions, and the strategic imperative for accelerated, patient-centric programs.
Scope & Segmentation Across the Clinical Trial Services Market
This report provides in-depth analysis across the Service Type segment, including:
- Analytical Testing Services: Advanced instrumentation and rapid molecular assay support.
- Bioanalytical Testing Services: High-throughput techniques for biomarker and molecular analysis.
- Clinical Trial Data Management Services: Unified platforms for electronic data capture and remote monitoring.
- Clinical Trial Management & Monitoring: End-to-end coordination, predictive analytics, and risk-based oversight.
- Medical Writing: Regulatory documentation and communications.
- Patient Recruitment & Retention: Digital outreach, community engagement, and retention campaigns.
- Regulatory & Safety Monitoring: Global compliance, timely reporting, and jurisdiction-specific monitoring.
- Safety & Pharmacovigilance: Ongoing assessment of drug safety for regulatory adherence.
The report further breaks down the market by Trial Phase, including all key preclinical and post-approval phases; Therapeutic Areas with a focus on oncology, cardiology, endocrinology, infectious diseases, and neurology; and End Users such as academic institutions, biotechnology firms, medical device manufacturers, and pharmaceutical companies.
Extensive regional coverage includes the Americas, Europe, Middle East & Africa, and Asia-Pacific. Analyses explore developments in North America, Latin America, Europe, Middle East, Africa, China, India, Japan, Australia, South Korea, and Southeast Asian markets. Featured company profiles highlight major industry players and recent corporate actions.
Key Takeaways: Clinical Trial Services Market
- Digital transformation and remote trial models are significantly enhancing operational efficiency and participant inclusion, while supporting regulatory requirements.
- The expansion of decentralized and adaptive studies enables real-time analytics and efficient patient engagement, improving outcomes and reducing timelines.
- Segment-specific differentiation—such as advanced analytical, data management, and patient support services—is helping providers address the diverse needs of sponsors and end users.
- Emerging regions in Asia-Pacific and the Middle East are becoming increasingly important, driven by supportive policies, digital health adoption, and growing biomanufacturing capabilities.
- Strategic alliances, investments in proprietary technology, and centers of excellence are central to the competitive approaches of leading firms in the clinical trial services market.
Tariff Impact: United States Tariffs in 2025
The introduction of United States tariffs in 2025 on laboratory equipment and medical devices is elevating operational expenses across contract research organizations. Service providers are mitigating cost pressures by diversifying suppliers, renegotiating agreements, and investing in domestic production. Sponsors are adapting by tightening budgets, adopting lab automation, and prioritizing risk-based strategies to maintain project viability. These measures signal a shift toward resilient global sourcing and increased operational scrutiny within the industry.
Methodology & Data Sources
This research integrates secondary data from scientific literature, regulatory databases, and industry publications with insights from primary interviews with clinical operations leaders and technology specialists. Quantitative market metrics are validated using data triangulation and scenario modeling. Rigorous peer reviews and quality checks ensure comprehensive, objective analysis supporting strategic decision-making.
Why This Report Matters for Senior Decision-Makers
- Gain actionable intelligence to inform strategic planning, supplier partnerships, and technology investments in clinical trial services.
- Understand the evolving technological, regulatory, and regional dynamics shaping your organization’s risk profile and opportunity landscape.
- Identify targeted growth and efficiency levers to create resilient, patient-focused trial operations aligned with global best practices.
Conclusion
Clinical trial services are at the center of pharmaceutical innovation, uniquely positioned to drive operational excellence. This report offers the clarity and foresight needed to strengthen competitive positioning and build adaptive, resilient research programs in a changing global environment.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Clinical Trial Services market report include:- IQVIA Holdings Inc.
- Parexel International Corporation
- Accenture plc
- Acurian, Inc.
- Caidya Inc.
- Celerion, Inc.
- Charles River Laboratories International, Inc.
- Clario, Inc.
- CMIC Holdings Co., Ltd.
- EPS International, Inc.
- ICON plc
- LGC Limited
- Clinipace Worldwide, Inc. by dMed Company Limited
- Medpace, Inc.
- Pharmaron, Inc.
- Thermo Fisher Scientific Inc
- Rho, Inc.
- PSI CRO AG
- Syneos Health, Inc.
- Synexus, Inc.
- Veristat, LLC
- Worldwide Clinical Trials, Inc.
- Wuxi AppTec Co., Ltd.
- Pharmaron, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 199 |
| Published | October 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 60.36 Billion |
| Forecasted Market Value ( USD | $ 106.13 Billion |
| Compound Annual Growth Rate | 8.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |