Speak directly to the analyst to clarify any post sales queries you may have.
Establishing the Critical Importance of Non-Opioid Therapeutics in Modern Pain Management, Patient-Centric Care Approaches, Strategies, and Outcomes
Non-opioid therapeutics have emerged as indispensable tools in the modern approach to pain management, reshaping how healthcare providers and patients perceive safety, efficacy, and long-term outcomes. With rising concerns over opioid dependency and regulatory scrutiny intensifying, the industry has pivoted toward solutions that deliver robust analgesia without the associated risk of abuse. Concurrently, patient-centric care models have fueled demand for therapies that integrate seamlessly into diverse treatment regimens, emphasizing tolerability alongside efficacy.Moreover, stakeholders across the healthcare ecosystem are increasingly prioritizing multidisciplinary pain pathways. This shift underscores the importance of non-opioid options in multimodal analgesia protocols, combining pharmacological and non-pharmacological strategies to optimize patient recovery. As clinical guidelines evolve to recommend reduced opioid exposure, the landscape for alternative analgesics is expanding, driven by both clinical imperatives and evolving patient expectations.
Consequently, pharmaceutical developers and healthcare institutions are investing in novel formulations and delivery technologies to enhance bioavailability, reduce dosing frequency, and minimize adverse events. These advancements not only bolster therapeutic performance but also align with broader healthcare objectives of improving quality of life, reducing hospital readmissions, and lowering overall treatment costs. In this context, understanding the critical importance of non-opioid therapeutics is essential for navigating the future of pain management.
Exploring Major Transformative Shifts Reshaping the Global Non-Opioid Therapeutics Landscape with Innovative Research Strategies and Technological Advancements
The non-opioid therapeutics landscape has experienced profound transformation, propelled by innovative research strategies and breakthroughs in drug discovery. Advances in molecular biology and computational modeling have enabled the identification of novel targets, leading to the development of therapies that modulate specific pain pathways without the systemic effects commonly associated with opioids. Furthermore, the integration of nanotechnology and sustained-release formulations is redefining how analgesics are delivered, offering more consistent therapeutic levels and reduced dosing intervals.In parallel, digital health platforms and remote monitoring tools are facilitating personalized treatment regimens, allowing clinicians to adjust dosing based on real-time patient feedback. This intersection of technology and therapeutics has accelerated the shift toward precision pain management, where data-driven insights inform both clinical decision-making and product development. Additionally, collaborations between biotech innovators and established pharmaceutical players have fostered an ecosystem of shared expertise, expediting the translation of early-stage discoveries into clinical candidates.
Regulatory bodies have also adapted to this evolving landscape, implementing frameworks that encourage expedited review of non-opioid alternatives. This has created an environment where agility and scientific rigor coexist, enabling stakeholders to respond swiftly to unmet clinical needs. As a result, the industry is witnessing an unprecedented wave of pipeline activity, characterized by targeted agents designed for conditions ranging from chronic neuropathic pain to inflammatory disorders.
Analyzing the Far-Reaching Cumulative Impact of Newly Imposed United States Tariffs on Non-Opioid Therapeutics Supply Chains, Costs, and Pricing Structures
The imposition of new United States tariffs on active pharmaceutical ingredients and formulation components has introduced significant complexity into non-opioid therapeutics supply chains and cost structures. Raw materials sourced from key international suppliers now face additional duties, leading manufacturers to reevaluate procurement strategies. Consequently, firms are increasingly exploring regional sourcing and near-shoring opportunities to mitigate exposure to trade regulation volatility.Moreover, the cumulative effect of these tariffs extends beyond direct input costs. Transportation expenses have risen as logistics providers adjust to redrawn trade routes and varying customs procedures. This has prompted companies to invest in supply chain optimization technologies and collaborative planning platforms, aiming to enhance visibility and reduce lead times. In turn, pricing strategies for end-market stakeholders must account for these elevated operational costs, compelling product teams to demonstrate clear value propositions.
In addition, the tariffs have spurred dialogue between industry associations and policymakers to advocate for tariff relief on critical medical supplies. While negotiations continue, organizations are diversifying supplier bases and forging strategic partnerships to ensure continuity of supply. Ultimately, navigating this tariff landscape requires a proactive approach to risk management and a willingness to adopt innovative sourcing models that safeguard both affordability and accessibility.
Revealing Critical Insights Across Drug Types, Administration Routes, Therapeutic Applications, End Use Settings, and Distribution Channels
A nuanced understanding of market segmentation is instrumental for identifying strategic growth opportunities across the non-opioid therapeutics sector. Within drug type classifications, acetaminophen remains foundational for mild to moderate pain, while antidepressants and antiepileptics are increasingly recognized for their neuropathic pain applications. Local anesthetics are experiencing refined formulations that enhance onset and duration, and nonsteroidal anti-inflammatory drugs continue to evolve with improved safety profiles.Turning to administration methods, intravenous delivery excels in acute settings where rapid onset is critical, whereas oral routes provide convenience for long-term outpatient management. Rectal formulations retain their utility in cases where oral access is compromised. In terms of therapeutic application, treatments for cancer-related pain demand specialized protocols distinct from chronic pain management, while inflammatory disorders and migraine care rely on agents that modulate specific biochemical pathways. Neurodegenerative disorder-related pain emerges as an area of growing focus, driven by aging demographics and unmet clinical needs.
End use contexts influence product development and commercialization strategies as well. Ambulatory surgical centers prioritize fast-acting agents to facilitate same-day discharges, diagnostic centers leverage anesthetic adjuncts for procedural comfort, and home care settings require user-friendly, self-administered formats. Hospitals and clinics maintain robust formularies to address diverse patient populations. Finally, distribution channels such as hospital pharmacies ensure controlled dispensation, online pharmacies cater to digital-savvy consumers seeking home delivery, and retail pharmacies sustain widespread accessibility. By integrating insights across these segmentation dimensions, stakeholders can tailor their approaches to align with evolving demand patterns.
Uncovering Regional Market Dynamics, Growth Drivers, and Emerging Challenges across the Americas, EMEA, and Asia-Pacific Non-Opioid Therapeutics Sectors
Regional dynamics exert a profound influence on non-opioid therapeutics adoption and innovation, with each geography presenting distinct drivers and obstacles. In the Americas, strong healthcare infrastructure, supportive reimbursement frameworks, and high patient awareness fuel demand for advanced analgesic solutions. Collaborative efforts between research institutions and commercial entities accelerate pipeline progression, while telemedicine expansion enhances patient access to specialist care.Conversely, Europe, the Middle East, and Africa present a tapestry of regulatory environments. Western European markets benefit from harmonized rules and progressive health technology assessments, whereas emerging markets in the Middle East and Africa contend with variable reimbursement policies and infrastructure limitations. Nonetheless, these regions are witnessing growing interest in non-opioid alternatives, prompted by government initiatives aimed at reducing opioid dependence and improving perioperative outcomes.
Meanwhile, the Asia-Pacific region is characterized by rapidly increasing healthcare expenditures, fueled by demographic shifts and rising chronic disease prevalence. Governments across Asia-Pacific are prioritizing domestic pharmaceutical manufacturing and fostering partnerships with multinational corporations. This collaborative approach, combined with a burgeoning middle class and expanding private healthcare networks, underscores the region's potential as a growth engine for next-generation non-opioid therapies.
Evaluating Leading Industry Players and Their Strategic Initiatives Driving Innovation, Partnerships, and Competitive Differentiation in Non-Opioid Therapeutic Solutions
The competitive landscape in non-opioid therapeutics is defined by established pharmaceutical giants and agile biotech innovators. Major multinational firms are leveraging extensive R&D infrastructures and deep regulatory expertise to advance late-stage clinical candidates. At the same time, specialized companies are carving niches through precision-targeted molecules and novel delivery platforms, often forging strategic alliances to scale commercialization.Partnerships between contract development organizations and pharmaceutical sponsors have become pivotal, enabling rapid iteration of formulations and accelerated time to market. In tandem, licensing agreements and co-development collaborations distribute risk while pooling scientific and commercial expertise. Beyond traditional collaborations, a new wave of venture-backed startups is attracting capital to develop first-in-class non-opioid analgesics, underscoring investor confidence in the sector's potential.
Furthermore, forward-looking companies are enhancing value propositions through digital companion tools, real-world evidence generation, and patient support programs. These integrated offerings strengthen market differentiation and foster loyalty among prescribers and patients alike. As a result, the competitive arena is evolving into an ecosystem where innovation, operational excellence, and strategic partnerships converge.
Providing Actionable Strategic Recommendations to Enhance Competitiveness, Innovation, and Market Access for Global Non-Opioid Therapeutics Industry Leaders
To maintain a leadership position in the non-opioid therapeutics market, organizations should intensify investment in targeted R&D while fostering cross-functional innovation. Embracing open innovation models can unlock external expertise and accelerate the development of breakthrough molecules. Additionally, strengthening supply chain resilience through diversified sourcing and digital tracking systems will safeguard against geopolitical and regulatory disruptions.Engaging proactively with regulatory agencies and health technology assessment bodies enables companies to anticipate requirements and streamline approval pathways. Moreover, adopting value-based pricing frameworks and demonstrating clear economic benefits will be critical for securing favorable reimbursement decisions. Simultaneously, integrating digital health platforms and real-world evidence initiatives enhances the credibility of product claims and supports long-term patient engagement.
Finally, forging strategic alliances with academic institutions, patient advocacy groups, and technology firms can amplify market reach and foster comprehensive care solutions. Through these concerted efforts, industry leaders can position themselves at the forefront of the evolving non-opioid therapeutics landscape, driving sustainable growth and delivering meaningful patient outcomes.
Detailing the Rigorous Research Methodology and Data Collection Framework Underpinning Comprehensive Non-Opioid Therapeutics Market Analysis
This analysis is grounded in a structured research methodology that integrates both primary and secondary data sources. Primary research included in-depth interviews with clinical experts, regulatory advisors, and supply chain specialists, complemented by stakeholder surveys that captured nuanced perspectives on market barriers and opportunities. These qualitative insights were triangulated with secondary information from peer-reviewed journals, patent registries, regulatory filings, and proprietary industry databases.Quantitative analysis utilized a combination of trend extrapolation, regression assessments, and scenario modeling to contextualize historical patterns and project potential strategic outcomes. Rigorous data validation processes ensured accuracy and consistency, while cross-verification with market participants enhanced reliability. Additionally, regional market intelligence teams provided localized insights, uncovering specific regulatory, reimbursement, and infrastructure considerations.
Analytical frameworks employed include SWOT evaluations and Porter's Five Forces, which elucidate competitive pressures, supplier dynamics, and customer bargaining scenarios. By leveraging this comprehensive research design, the report delivers robust, actionable insights that reflect the complexities of the non-opioid therapeutics landscape.
Summarizing Core Insights and Implications for Stakeholders in the Evolving Non-Opioid Therapeutics Landscape with Forward-Looking Perspectives
The non-opioid therapeutics sector stands at a pivotal juncture, driven by evolving clinical paradigms, regulatory imperatives, and patient demand for safer pain management options. Stakeholders must navigate an intricate interplay of supply chain challenges, regional market dynamics, and intensifying competition. Yet, the confluence of technological innovation, collaborative partnerships, and data-driven decision-making creates a roadmap for sustainable growth.By integrating targeted segmentation insights with an understanding of global tariff impacts and regional nuances, industry players can tailor their strategies to emerging opportunities. Simultaneously, actionable recommendations offer a blueprint for enhancing R&D productivity, fortifying supply chains, and optimizing market access. As the field continues to advance, the ability to adapt swiftly and leverage cross-sector expertise will distinguish leading organizations.
In conclusion, embracing a holistic, patient-centric approach-supported by rigorous research and strategic agility-will be essential for delivering transformative non-opioid therapies. The insights presented herein serve as a foundation for informed decision-making, preparing stakeholders to meet the evolving needs of patients and healthcare systems alike.
Market Segmentation & Coverage
This research report forecasts revenues and analyzes trends in each of the following sub-segmentations:- Drug Type
- Acetaminophen
- Antidepressants
- Antiepileptics
- Local Anesthetics
- Nonsteroidal Anti-Inflammatory Drugs
- Route of Administration
- Intravenous
- Oral
- Rectal
- Application
- Cancer Related Pain
- Chronic Pain
- Inflammatory Disorders
- Migraine
- Neurodegenerative Disorders
- End Use
- Ambulatory Surgical Centers
- Diagnostic Centers
- Home Care Settings
- Hospitals & Clinics
- Distribution Channel
- Hospital Pharmacies
- Online Pharmacies
- Retail Pharmacies
- Americas
- North America
- United States
- Canada
- Mexico
- Latin America
- Brazil
- Argentina
- Chile
- Colombia
- Peru
- North America
- Europe, Middle East & Africa
- Europe
- United Kingdom
- Germany
- France
- Russia
- Italy
- Spain
- Netherlands
- Sweden
- Poland
- Switzerland
- Middle East
- United Arab Emirates
- Saudi Arabia
- Qatar
- Turkey
- Israel
- Africa
- South Africa
- Nigeria
- Egypt
- Kenya
- Europe
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- Indonesia
- Thailand
- Malaysia
- Singapore
- Taiwan
- AbbVie Inc.
- GlaxoSmithKline plc
- Acorda Therapeutics, Inc.
- Allay Therapeutics Inc.
- Alnylam Pharmaceuticals, Inc.
- Biogen Inc.
- Centrexion Therapeutics,
- Concentric Analgesics, Inc.
- Confo Therapeutics NV
- Durect Corporation
- Eli Lilly and Company
- Latigo Biotherapeutics, Inc.
- Lexicon Pharmaceuticals, Inc.
- Liquidia Corporation
- McNeil-PPC, Inc. by Johnson & Johnson Inc.
- Mylan N.V. by Viatris Inc.
- Neumentum Inc.
- SiteOne Therapeutics, Inc.
- Sun Pharmaceutical Industries Limited
- Tris Pharma, Inc.
- Vertex Pharmaceuticals Incorporated
- Pfizer Inc.
- Amgen Inc.
- Cara Therapeutics, Inc.
- Bioelectronics Corporation
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Non-Opioid Therapeutics Market report include:- AbbVie Inc.
- GlaxoSmithKline plc
- Acorda Therapeutics, Inc.
- Allay Therapeutics Inc.
- Alnylam Pharmaceuticals, Inc.
- Biogen Inc.
- Centrexion Therapeutics,
- Concentric Analgesics, Inc.
- Confo Therapeutics NV
- Durect Corporation
- Eli Lilly and Company
- Latigo Biotherapeutics, Inc.
- Lexicon Pharmaceuticals, Inc.
- Liquidia Corporation
- McNeil-PPC, Inc. by Johnson & Johnson Inc.
- Mylan N.V. by Viatris Inc.
- Neumentum Inc.
- SiteOne Therapeutics, Inc.
- Sun Pharmaceutical Industries Limited
- Tris Pharma, Inc.
- Vertex Pharmaceuticals Incorporated
- Pfizer Inc.
- Amgen Inc.
- Cara Therapeutics, Inc.
- Bioelectronics Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 197 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
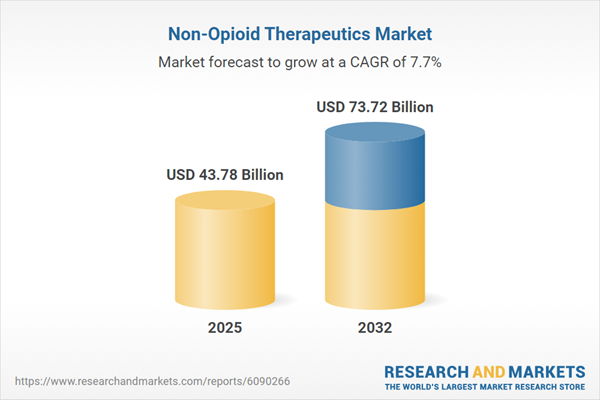
| Estimated Market Value ( USD | $ 43.78 Billion |
| Forecasted Market Value ( USD | $ 73.72 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |