Speak directly to the analyst to clarify any post sales queries you may have.
The siRNA Therapeutics Market is evolving rapidly, driven by advances in delivery platforms, regulatory clarity, and a shift in strategic priorities among leading pharmaceutical and biotechnology organizations. Senior decision-makers must navigate a landscape marked by intensifying competition, operational complexity, and dynamic policy changes—requiring clear insights to make well-informed investments and strategy choices.
Market Snapshot: Strong Growth and Shifting Competitive Landscape
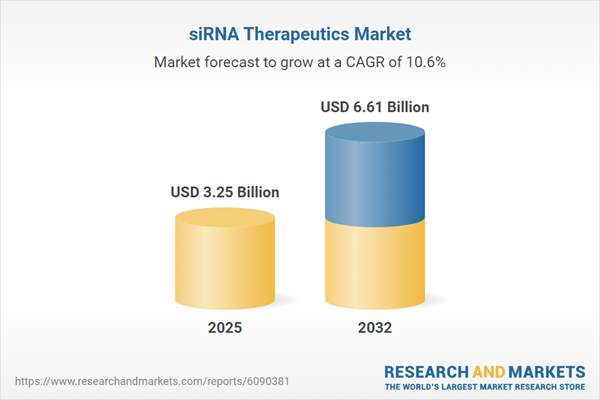
The siRNA therapeutics sector experienced significant growth, increasing from USD 2.95 billion in 2024 to USD 3.25 billion in 2025. Analysts expect continued acceleration at a compound annual growth rate (CAGR) of 10.61%, forecasting a value of USD 6.61 billion by 2032. This expansion is underpinned by clinical validation, improved delivery modalities, and broader adoption of RNA interference therapies across diverse disease areas. Investors and strategic partners are increasingly drawn to assets capable of solving dosing and delivery challenges, while payers and health systems are focused on the long-term value associated with less frequent dosing regimens.
Scope & Segmentation: siRNA Therapeutics Market
- Drug Agents: Givosiran, Inclisiran, Lumasiran, Nedosiran, Patisiran, Vutrisiran
- Delivery Systems: Lipid nanoparticles, peptide delivery systems, polymer conjugates
- Route of Administration: Intravenous (IV), subcutaneous
- Therapeutic Categories: Cardiovascular disorders, immunological disorders, infectious diseases (bacterial and viral), metabolic disorders, neurological disorders (Alzheimer's disease, Parkinson's disease), oncology (hematologic malignancies such as leukemia and lymphoma, solid tumors including breast, lung, and prostate cancer)
- End-Users: Contract research organizations, hospitals and clinics, pharmaceutical companies, research and academic institutes
- Regional Coverage: Americas (United States, Canada, Mexico, Brazil, Argentina, Chile, Colombia, Peru), Europe, Middle East & Africa (United Kingdom, Germany, France, Russia, Italy, Spain, Netherlands, Sweden, Poland, Switzerland, United Arab Emirates, Saudi Arabia, Qatar, Turkey, Israel, South Africa, Nigeria, Egypt, Kenya), Asia-Pacific (China, India, Japan, Australia, South Korea, Indonesia, Thailand, Malaysia, Singapore, Taiwan)
- Key Companies Analyzed: Alnylam Pharmaceuticals Inc., Alys Pharmaceuticals Inc., Arbutus Biopharma Corporation, Arcturus Therapeutics (by Alcobra Ltd.), Aro Biotherapeutics Co., Arrowhead Pharmaceuticals Inc., Atalanta Therapeutics, Biocon Ltd., e-Therapeutics Plc, Eli Lilly and Company, Ionis Pharmaceuticals Inc., Merck & Co. Inc., Moderna Therapeutics, Novartis AG, Novo Nordisk A/S, Phio Pharmaceuticals, Quark Pharmaceuticals, Risen Pharma Tech Co Ltd, Roche Holding AG, Shanghai Argo Biopharmaceutical, Silence Therapeutics plc, Sirius Therapeutics Inc., Sirnaomics Inc., Suzhou Sanegene Bio Inc., Synerk Inc., Thermo Fisher Scientific Inc., Vir Biotechnology, Wave Life Sciences USA, Inc.
Key Takeaways for Senior Decision-Makers
- Advancements in delivery technologies, including lipid nanoparticles and conjugates, are enabling drug developers to target disease areas beyond the liver, creating new commercial opportunities.
- Strategic partnerships and licensing between large pharmaceutical firms and platform specialists are accelerating late-stage development and global market expansion.
- Operational models are transitioning toward scalable, quality-driven manufacturing and supply networks, supported by investment in dedicated oligonucleotide production and robust quality management systems.
- Payer adoption and commercial success increasingly depend on value demonstration, with subscription-style and outcome-based models shaping future reimbursement discussions.
- Scientific innovation, cross-platform technology transfer, and next-generation delivery systems are amplifying therapeutic reach and addressing long-standing dosing and adherence barriers.
- Regional diversity in regulatory requirements, manufacturing capabilities, and payer landscapes demand flexible commercialization and evidence-generation strategies across the Americas, EMEA, and Asia-Pacific.
Tariff Impact and Supply Chain Considerations
Recent tariff adjustments and policy changes in the United States are reshaping global supply chain and sourcing strategies for siRNA therapeutics. Increased landed costs, longer lead times, and added administrative complexity are impacting operational efficiency. Companies are considering localized manufacturing and nearshoring to mitigate risk, but doing so requires fresh investment in facilities and quality systems. Smaller innovators may face disproportionate pressures, necessitating closer collaboration with suppliers and early regulatory engagement to secure essential clinical and commercial supplies.
Methodology & Data Sources
This analysis is based on structured interviews with drug developers, delivery platform specialists, CDMO leaders, regulatory consultants, and payers. The research is supported by detailed regulatory reviews, patent analyses, clinical pipeline mapping, and targeted scenario validation to assess operational implications of policy changes and supply chain shifts.
Why This Report Matters
- Provides a comprehensive view enabling executives to define scalable investment and partnership strategies across the siRNA value chain
- Equips stakeholders to proactively address emerging regulatory, manufacturing, and policy risks across major regional markets
- Empowers leaders to benchmark operational resilience and innovation priorities for sustainable commercial success
Conclusion
siRNA therapeutics are shifting from proof-of-concept to clinical reality, propelled by delivery innovation and regulatory progress. Companies that prioritize supply chain resilience, cross-sector partnerships, and adaptable business models will be best positioned for future growth and broad clinical impact.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this siRNA Therapeutics market report include:- Alnylam Pharmaceuticals Inc.
- Alys Pharmaceuticals Inc.
- Arbutus Biopharma Corporation
- Arcturus Therapeutics, Inc. by Alcobra Ltd.
- Aro Biotherapeutics Co.
- Arrowhead Pharmaceuticals Inc.
- Atalanta Therapeutics
- Biocon Ltd.
- e-Therapeutics Plc
- Eli Lilly and Company
- Ionis Pharmaceuticals Inc.
- Merck & Co. Inc.
- Moderna Therapeutics
- Novartis AG
- Novo Nordisk A/S
- Phio Pharmaceuticals, Inc.
- Quark Pharmaceuticals inc.
- Risen Pharma Tech Co Ltd
- Roche Holding AG
- Shanghai Argo Biopharmaceutical Co., Ltd.
- Silence Therapeutics plc
- Sirius Therapeutics Inc.
- Sirnaomics Inc.
- Suzhou Sanegene Bio Inc.
- Synerk Inc.
- Thermo Fisher Scientific Inc.
- Vir Biotechnology, Inc.
- Wave Life Sciences USA, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 195 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 3.25 Billion |
| Forecasted Market Value ( USD | $ 6.61 Billion |
| Compound Annual Growth Rate | 10.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 29 |