Speak directly to the analyst to clarify any post sales queries you may have.
The peptide purification system market is advancing rapidly, driven by increasing requirements for high-purity peptides in pharmaceutical, diagnostics, and biotechnology applications. This report delivers a decisive view of current industry shifts, enabling senior leaders to better anticipate challenges and opportunities.
Market Snapshot: Growth and Opportunity in Peptide Purification Systems
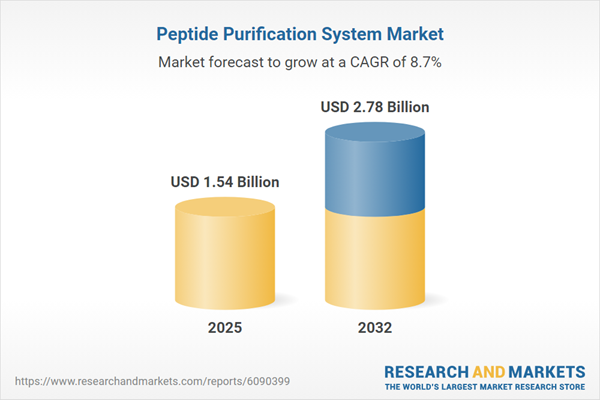
The peptide purification system market grew from USD 1.42 billion in 2024 to USD 1.54 billion in 2025. It is expected to continue growing at a compound annual growth rate of 8.70%, reaching USD 2.78 billion by 2032. Increasingly stringent regulatory standards and the urgent need for process efficiency in drug discovery and clinical research are powering sector expansion. Surging global R&D investments and a shift to automated, data-driven laboratory operations further accelerate demand. Organizations across North America, Europe, Asia-Pacific, and emerging regions are investing in next-generation peptide separation technologies to secure competitive advantages.
Scope & Segmentation Across the Peptide Purification System Market
- Purification Technologies: Chromatography, including affinity chromatography, ion exchange chromatography, reversed-phase chromatography, and size exclusion chromatography. Membrane filtration platforms supporting high-throughput buffer exchange and concentration are also included.
- Peptide Types: Natural peptides and synthetic peptides, each with distinct process and equipment considerations.
- End Users: Academic and research institutions, contract research organizations, diagnostics and clinical laboratories, and pharmaceutical and biotechnology companies.
- Research Applications: Drug discovery, immunology, proteomics, and structural biology, reflecting diverse purification needs in both exploratory and regulated environments.
- Scale of Operations: Large-scale, medium-scale, and small-scale systems to address commercial production, process development, and analytical validation workflows.
- Geographical Coverage: Americas (North America, Latin America), Europe, Middle East & Africa, and Asia-Pacific, each with regionally nuanced drivers for peptide purification investment.
- Company Landscape: Coverage and analysis of players such as Advion, Inc., Agilent Technologies, Inc., Biotage AB, Büchi Labortechnik AG, Cell Signaling Technology, Inc., Danaher Corporation, DuPont de Nemours, Inc., Gilson, Inc., Good Science (Tianjin) Instrument Technologies Co.,Ltd, Gyros Protein Technologies AB, JASCO International, Inc., Knauer Wissenschaftliche Geräte GmbH, Phenomenex, Inc., Sepax Technologies, Inc., Shimadzu Corporation, Teledyne Technologies, Inc., Thermo Fisher Scientific Inc., Tosoh Corporation, Waters Corporation, and YMC Co., Ltd.
Key Takeaways for Senior Decision-Makers
- Peptide purification continues its evolution, transitioning from traditional chromatography toward modular systems that integrate membrane filtration, automation, and digital analytics for seamless R&D workflows.
- Stakeholders are re-evaluating long-standing purification infrastructure to blend the reliability of legacy techniques with data-centric, scalable solutions that address both current and future operational demands.
- Regulatory and quality expectations are raising the bar on process reproducibility and validation requirements, prompting robust investments in system upgrades and digital monitoring for compliance.
- Regional investment patterns reveal growth opportunities, particularly where academic, pharmaceutical, and contract research activities are expanding or where government incentives for biotechnology manufacturing exist.
- Collaboration across supply chains and alliances with technology developers are enabling more agile responses to changing customer needs, especially within fast-tracked drug development pipelines.
Impact of United States Tariffs on Supply Chains and Cost Structures
Recently enacted United States tariffs have created significant cost challenges for peptide purification operations reliant on imported chromatographic resins, specialty solvents, and single-use filtration modules. Many organizations are diversifying supplier portfolios, securing domestic or regional alternatives, and renegotiating procurement contracts to include tariff-adjustment clauses. While initial disruption may extend qualification cycles, these adjustments are expected to enhance supply continuity and price flexibility, and may prompt broader adoption of transparent, data-driven supply-chain management.
Methodology & Data Sources
This report is underpinned by a rigorous methodology, combining secondary research from peer-reviewed literature, patents, white papers, press releases, and regulatory commentary with primary research via interviews and surveys among peptide purification system users, procurement leads, and C-suite executives. Analytical frameworks and segment-level triangulation were performed to validate insights and ensure relevance for strategic planning.
Why This Report Matters to the Life Sciences Industry
- Enables more confident investment decisions by delivering a clearly segmented overview of technology options, market trends, and region-specific growth factors for peptide purification systems.
- Provides actionable guidance to optimize procurement strategies in response to tariff policy, regulatory change, and evolving customer requirements.
- Supports informed product development and partnership negotiations for organizations seeking to capitalize on innovation in purification and process analytics.
Conclusion
Senior executives and technology leaders can leverage these findings to navigate industry transformation, enhance purification outcomes, and safeguard supply chains. This report offers actionable intelligence to support sustained progress in peptide-based therapeutics and research.
Table of Contents
3. Executive Summary
4. Market Overview
7. Cumulative Impact of Artificial Intelligence 2025
Companies Mentioned
The companies profiled in this Peptide Purification System market report include:- Advion, Inc.
- Agilent Technologies, Inc.
- Biotage AB
- Büchi Labortechnik AG
- Cell Signaling Technology, Inc.
- Danaher corporation
- DuPont de Nemours, Inc.
- Gilson, Inc.
- Good Science (Tianjin) Instrument Technologies Co.,Ltd
- Gyros Protein Technologies AB
- JASCO International, Inc.
- Knauer Wissenschaftliche Geräte GmbH
- Phenomenex, Inc.
- Sepax Technologies, Inc.
- Shimadzu Corporation
- Teledyne Technologies, Inc.
- Thermo Fisher Scientific Inc.
- Tosoh Corporation
- Waters Corporation
- YMC Co., Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | November 2025 |
| Forecast Period | 2025 - 2032 |
| Estimated Market Value ( USD | $ 1.54 Billion |
| Forecasted Market Value ( USD | $ 2.78 Billion |
| Compound Annual Growth Rate | 8.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |







![Single-use Bioprocessing Market by Product (Equipment [Bioreactors, Filtration, Chromatography], Consumables [Filters, Bags, Assemblies, Sensors]), Application (Storage, Mixing), Workflow (Upstream), Molecule Type (mAbs, Vaccines) - Global Forecast to 2030 - Product Image](http://www.researchandmarkets.com/product_images/12020/12020910_60px_jpg/singleuse_bioprocessing_market.jpg)
