Global Clinical Laboratory Tests Market - Key Trends & Drivers Summarized
Why Are Clinical Laboratory Tests Central to Disease Detection, Personalized Medicine, and Population Health Management?
Clinical laboratory tests are foundational to modern healthcare, providing critical insights that guide diagnosis, treatment decisions, and disease monitoring across a broad spectrum of medical conditions. From routine blood work and metabolic panels to advanced molecular diagnostics and genetic screening, these tests support over 70% of clinical decisions, making them indispensable in both acute and preventive care settings. Their relevance continues to grow as healthcare systems shift toward value-based care, early intervention, and personalized treatment strategies.Increasing prevalence of chronic diseases, aging populations, and the need for rapid infectious disease diagnostics are driving demand for more comprehensive and timely laboratory testing. In both hospital-based and outpatient environments, clinical lab tests are key to monitoring patient outcomes, managing therapeutic efficacy, and enabling risk stratification - especially in oncology, cardiology, endocrinology, and infectious diseases.
How Are Test Automation, Multiplex Platforms, and Data Integration Advancing Laboratory Efficiency and Accuracy?
Technological advancements are transforming clinical laboratories into high-throughput, precision-driven diagnostic hubs. Automation and robotics are reducing manual errors, standardizing workflows, and increasing testing capacity in centralized labs. Multiplex and syndromic testing platforms are enabling simultaneous detection of multiple biomarkers from a single sample, improving diagnostic speed and clinical relevance - especially in respiratory panels, STI testing, and cancer biomarker profiling.Molecular diagnostics, including PCR, next-generation sequencing (NGS), and isothermal amplification, are becoming increasingly integrated into routine lab operations, supporting earlier and more precise detection of genetic disorders, infectious agents, and treatment-resistant strains. Immunoassay innovations and lab-on-a-chip systems are further enhancing sensitivity, miniaturization, and turnaround time.
Digitalization and interoperability with electronic health records (EHRs) are allowing for seamless test ordering, results reporting, and clinical decision support. Advanced analytics and AI-driven interpretation tools are also being incorporated to reduce diagnostic uncertainty and support evidence-based medicine, particularly in complex or data-intensive test results.
Which Healthcare Settings and Regional Health Trends Are Influencing Clinical Lab Test Demand?
Clinical laboratory tests are performed across hospitals, diagnostic centers, reference labs, outpatient clinics, and point-of-care (POC) settings. While centralized labs remain dominant due to economies of scale and test variety, decentralized and near-patient testing is gaining traction in emergency care, rural health delivery, and chronic disease monitoring.North America and Europe represent mature markets with advanced infrastructure, insurance coverage, and regulatory compliance. Asia-Pacific is showing strong growth, driven by expanding healthcare access, increased health screening awareness, and government investment in diagnostics capacity. In Latin America, the Middle East, and Africa, rising chronic disease burden and infrastructure development are propelling demand for both basic and advanced lab tests.
Global health initiatives, public-private partnerships, and pandemic preparedness programs are also supporting market expansion in underdiagnosed populations and underserved geographies.
What Are the Factors Driving Growth in the Clinical Laboratory Tests Market?
The clinical laboratory tests market is growing as diagnostics become increasingly central to proactive healthcare delivery, precision medicine, and disease surveillance. The expanding role of lab testing in clinical decision-making, regulatory compliance, and public health initiatives underscores its strategic value in modern medical systems.Key growth drivers include rising disease burden, demand for early diagnosis and preventive screening, technological innovation in test platforms, increased healthcare spending, and growing patient awareness. The integration of AI, automation, and molecular insights into traditional lab workflows is further enhancing accuracy, speed, and clinical utility.
As global health systems prioritize diagnostics-driven care models, could clinical laboratory tests evolve into the definitive interface between population health intelligence and personalized treatment pathways?
Report Scope
The report analyzes the Clinical Laboratory Tests market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Test Type (Clinical Chemistry, Medical Microbiology, Hematology Testing, Immunology Testing, Cytology Testing, Drug of Abuse Testing, Other Test Types); Service Provider (Hospital-based Laboratories, Standalone Laboratories, Clinic-based Laboratories).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Clinical Chemistry segment, which is expected to reach US$123.5 Billion by 2030 with a CAGR of a 7.5%. The Medical Microbiology segment is also set to grow at 6.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $74 Billion in 2024, and China, forecasted to grow at an impressive 10% CAGR to reach $80.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Clinical Laboratory Tests Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Clinical Laboratory Tests Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Clinical Laboratory Tests Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Carbon Streaming Corporation, Carbon Trust, Carbonplace, CDP (Carbon Disclosure Project), Climate Impact Partners and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Clinical Laboratory Tests market report include:
- Abbott Laboratories
- ARUP Laboratories
- Bio-Rad Laboratories
- bioMérieux S.A.
- Charles River Laboratories
- Clinical Reference Laboratory, Inc.
- Eurofins Scientific
- Fresenius Medical Care AG & Co. KGaA
- Genoptix, Inc.
- Healthscope Limited
- Laboratory Corporation of America Holdings (LabCorp)
- Merck KGaA
- NeoGenomics Laboratories
- OPKO Health, Inc.
- QIAGEN N.V.
- Quest Diagnostics Incorporated
- Siemens Healthineers
- Sonic Healthcare Limited
- SYNLAB International GmbH
- Unilabs
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- ARUP Laboratories
- Bio-Rad Laboratories
- bioMérieux S.A.
- Charles River Laboratories
- Clinical Reference Laboratory, Inc.
- Eurofins Scientific
- Fresenius Medical Care AG & Co. KGaA
- Genoptix, Inc.
- Healthscope Limited
- Laboratory Corporation of America Holdings (LabCorp)
- Merck KGaA
- NeoGenomics Laboratories
- OPKO Health, Inc.
- QIAGEN N.V.
- Quest Diagnostics Incorporated
- Siemens Healthineers
- Sonic Healthcare Limited
- SYNLAB International GmbH
- Unilabs
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 292 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
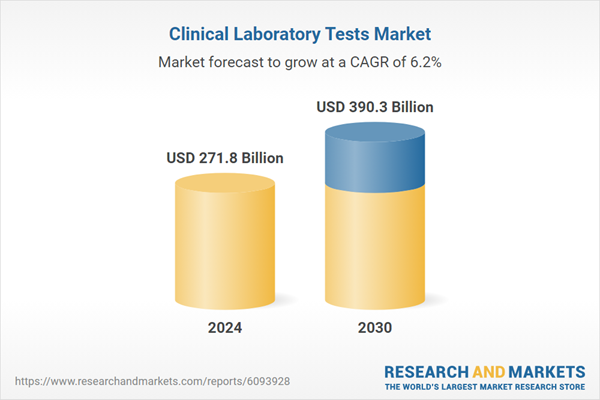
| Estimated Market Value ( USD | $ 271.8 Billion |
| Forecasted Market Value ( USD | $ 390.3 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |