Global Biomanufacturing Viral Detection and Quantifications Market - Key Trends & Drivers Summarized
Why Is Viral Detection and Quantification Crucial in Biomanufacturing Today?
In the field of biomanufacturing, viral detection and quantification have become indispensable processes, ensuring the safety, quality, and efficacy of biologically-derived products. Biologics, such as monoclonal antibodies, gene therapies, vaccines, and recombinant proteins, are often produced using living cell systems that can be vulnerable to viral contamination during development, production, or packaging stages. Even trace amounts of adventitious viruses - whether from raw materials, host cells, or the production environment - can compromise entire product batches, lead to regulatory non-compliance, and pose serious health risks to patients. Therefore, comprehensive viral safety strategies involving both detection (identifying the presence of viruses) and quantification (measuring viral load or titer) are critical to biomanufacturing workflows. These processes help ensure that final biopharmaceutical products are free from both known and emerging viral contaminants. Regulatory agencies such as the FDA, EMA, and WHO mandate rigorous viral safety testing and validation throughout the development lifecycle, making this area not just a technical necessity but a legal imperative. Moreover, with the rise of complex biologics and personalized therapies - especially cell and gene therapies - the sensitivity and accuracy of viral detection systems must evolve accordingly. In this environment, reliable viral monitoring has become a cornerstone of manufacturing assurance, product consistency, and public health protection in the biopharma sector.How Are Innovations in Detection Technologies Improving Viral Safety in Biomanufacturing?
Technological advancements are significantly enhancing the accuracy, speed, and sensitivity of viral detection and quantification in biomanufacturing. Traditional methods such as in vivo animal testing and in vitro cell culture assays, while still important, are increasingly being complemented or replaced by modern molecular techniques. Real-time quantitative polymerase chain reaction (qPCR) and digital PCR have become gold standards for their ability to detect and quantify specific viral genomes with high precision and rapid turnaround times. Additionally, next-generation sequencing (NGS) technologies are revolutionizing viral detection by enabling unbiased identification of both known and novel viral contaminants, thereby strengthening safety in early and late-stage manufacturing. These techniques offer high-throughput, deep-sequencing capabilities that allow for comprehensive viral surveillance at the genetic level. Advances in bioinformatics are further empowering researchers to analyze massive datasets quickly, identifying viral signatures even at very low concentrations. Other innovations include biosensors using surface plasmon resonance (SPR), immunoassays with enhanced monoclonal antibody specificity, and mass spectrometry techniques that profile viral proteins. Automation and digitalization of testing workflows are reducing manual errors and ensuring consistency across batches. Artificial intelligence is also being leveraged to predict contamination risks and optimize detection protocols. These cutting-edge technologies not only improve detection limits and reduce false positives/negatives but also facilitate real-time, in-line monitoring during biomanufacturing, enabling faster corrective actions. As a result, these innovations are raising the standard of viral safety across the biomanufacturing industry and creating robust systems to support high-stakes therapeutic production.Which Sectors and Geographies Are Leading the Push for Viral Safety in Biomanufacturing?
The demand for effective viral detection and quantification in biomanufacturing is being driven by multiple sectors, with the pharmaceutical and biotechnology industries at the forefront. Companies producing biologics - especially monoclonal antibodies, recombinant proteins, cell and gene therapies, and vaccines - have the highest need for stringent viral monitoring protocols. This is particularly true for gene therapy vectors like AAVs (adeno-associated viruses) and lentiviruses, which require exact quantification to ensure safety, dose consistency, and therapeutic efficacy. CDMOs (contract development and manufacturing organizations) also play a key role, offering viral safety testing as a critical service for smaller biotech firms and clinical-stage developers. In addition to pharma, the diagnostics industry is increasingly focused on viral quantification, especially for products involving viral reagents or derived from cell cultures. Geographically, North America leads the market, bolstered by its advanced biomanufacturing infrastructure, strong regulatory oversight, and heavy investments in biologic development. The U.S., home to the FDA and many leading biotech firms, sets global standards for viral safety. Europe follows closely, driven by the EMA's stringent quality regulations and a mature biologics ecosystem in countries like Germany, the UK, and Switzerland. Asia-Pacific, particularly China, South Korea, India, and Japan, is experiencing rapid growth in biomanufacturing capabilities and is investing heavily in quality control to meet global export standards. These regions are also ramping up domestic biologic production, further fueling the demand for viral safety assurance. Globally, the expansion of pandemic preparedness initiatives and the rise of biologics across emerging markets are broadening the geographic scope of demand for viral detection and quantification.What Is Fueling the Growth in the Global Biomanufacturing Viral Detection and Quantifications Market?
The growth in the global biomanufacturing viral detection and quantifications market is driven by several key factors: the increasing production of complex biologics, rising regulatory scrutiny, growing incidence of viral outbreaks, and ongoing advancements in detection technologies. One of the primary growth drivers is the expanding pipeline of biologic drugs and advanced therapies - particularly gene and cell therapies - which require meticulous viral safety monitoring at every stage. These treatments often involve viral vectors or are produced in viral-prone environments, making sensitive detection and precise quantification absolutely critical. Regulatory agencies are tightening viral safety requirements, pushing manufacturers to adopt the latest, most robust testing methodologies. High-profile recalls and contamination incidents have also increased awareness of viral risks, leading to greater investment in preventive quality control. Another major growth factor is the global focus on pandemic preparedness and biosurveillance following the COVID-19 crisis, which exposed vulnerabilities in biological supply chains. This has accelerated demand for fast, scalable viral detection systems not just for finished products, but also for raw materials and process intermediates. Technological innovation - particularly in digital PCR, NGS, and AI-based analytics - is lowering testing costs, improving accessibility, and making it easier to integrate viral monitoring into continuous bioprocessing environments. Furthermore, the globalization of biomanufacturing and the rise of CDMOs are extending viral detection requirements to new markets and facilities worldwide. These trends are also supported by strategic partnerships between diagnostics companies, regulatory bodies, and academic institutions aimed at standardizing protocols and developing universal detection frameworks. Altogether, these drivers are ensuring that viral detection and quantification remain indispensable to the safe, scalable, and compliant production of biologics in the years ahead.Report Scope
The report analyzes the Biomanufacturing Viral Detection and Quantifications market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Offering (Consumables Offering, Instruments Offering, Services Offering); Technology (PCR Technology, ELISA Technology, Flow Cytometry Technology, Plaque Assay Technology, Other Technologies); Application (Blood & Blood Products Manufacturing Application, Vaccines & Therapeutics Manufacturing Application, Cellular & Gene Therapy Products Manufacturing Application, Stem Cell Products Manufacturing Application, Tissue & Tissue Products Manufacturing Application); End-Use (Life Science Companies End-Use, Testing Laboratories End-Use, CROs & CDMOs End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Consumables Offering segment, which is expected to reach US$464.1 Million by 2030 with a CAGR of a 5.3%. The Instruments Offering segment is also set to grow at 8.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $149.2 Million in 2024, and China, forecasted to grow at an impressive 9.8% CAGR to reach $161.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Biomanufacturing Viral Detection and Quantifications Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Biomanufacturing Viral Detection and Quantifications Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Biomanufacturing Viral Detection and Quantifications Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Amgen Inc., Biocon Limited, BioNTech SE, Boehringer Ingelheim GmbH and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 43 companies featured in this Biomanufacturing Viral Detection and Quantifications market report include:
- Abingdon Health
- Agilent Technologies, Inc.
- Ayoxxa Biosystems
- Bio-Rad Laboratories, Inc.
- Charles River Laboratories
- Creative Diagnostics
- Danaher Corporation
- DiaSorin S.p.A.
- Eurogentec
- FUJIFILM Diosynth Biotechnologies
- GenScript Biotech Corporation
- Lonza Group Ltd.
- Merck KGaA
- New England Biolabs
- Norgen Biotek Corp.
- PerkinElmer, Inc.
- Promega Corporation
- QIAGEN N.V.
- Sartorius AG
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abingdon Health
- Agilent Technologies, Inc.
- Ayoxxa Biosystems
- Bio-Rad Laboratories, Inc.
- Charles River Laboratories
- Creative Diagnostics
- Danaher Corporation
- DiaSorin S.p.A.
- Eurogentec
- FUJIFILM Diosynth Biotechnologies
- GenScript Biotech Corporation
- Lonza Group Ltd.
- Merck KGaA
- New England Biolabs
- Norgen Biotek Corp.
- PerkinElmer, Inc.
- Promega Corporation
- QIAGEN N.V.
- Sartorius AG
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 484 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
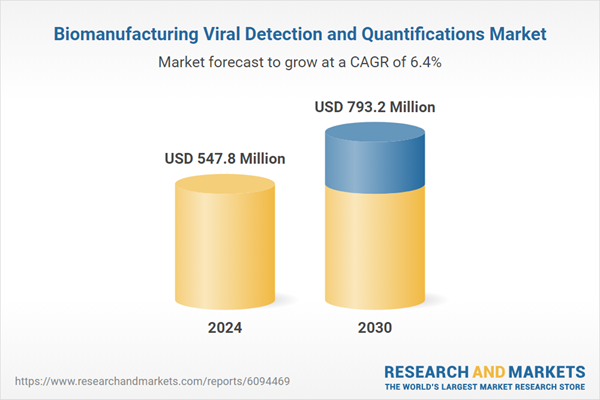
| Estimated Market Value ( USD | $ 547.8 Million |
| Forecasted Market Value ( USD | $ 793.2 Million |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |