Global Balloon Aortic Valvuloplasty Market - Key Trends & Drivers Summarized
What Is Driving Renewed Interest in Balloon Aortic Valvuloplasty in the Cardiovascular Space?
Balloon Aortic Valvuloplasty (BAV) is experiencing a significant resurgence in global interest, primarily driven by evolving clinical needs and expanding indications for use. Traditionally viewed as a palliative intervention for patients ineligible for surgical or transcatheter aortic valve replacement (TAVR), BAV has recently emerged as a strategic tool in pre-TAVR optimization, valve assessment, and as a bridging therapy for high-risk patients. The growing global burden of aortic stenosis, particularly among aging populations in both developed and emerging economies, underscores the demand for minimally invasive interventions that offer symptomatic relief with a lower procedural risk. As BAV techniques improve, procedural safety has seen marked advancement due to better imaging guidance, catheter designs, and perioperative monitoring. Furthermore, the integration of BAV in hybrid cardiac procedures has widened its clinical utility. In nations where healthcare resources are limited, BAV remains a viable interim solution for symptom relief in patients awaiting definitive valve replacement. Technological innovation in low-profile balloon catheters and refined hemodynamic monitoring has contributed to higher success rates and reduced complications. As such, clinical perceptions surrounding BAV are shifting from viewing it as a last-resort option to recognizing it as a critical component of comprehensive aortic stenosis management, particularly in fragile patients who are unable to undergo definitive procedures immediately.Why Is There a Regional Disparity in Adoption Rates and Procedural Volumes?
The global adoption of Balloon Aortic Valvuloplasty varies considerably across regions, influenced by disparities in healthcare infrastructure, availability of advanced cardiovascular facilities, and practitioner training. In high-income countries such as the United States, Germany, and Japan, BAV is increasingly employed not only in emergency interventions but also in preoperative planning for TAVR and surgical procedures. The sophistication of interventional cardiology centers in these regions, alongside supportive reimbursement policies and widespread access to high-resolution fluoroscopy and echocardiographic imaging, enhances the feasibility and safety of BAV. Conversely, in lower-middle-income countries across Latin America, Africa, and parts of Southeast Asia, BAV remains underutilized due to limited access to trained cardiologists, high procedural costs, and lack of awareness among referring physicians. However, emerging initiatives in telemedicine and mobile cath labs are slowly improving access in underserved areas. Moreover, international partnerships and philanthropic investments are facilitating training and technology transfers, allowing for greater procedural standardization. Clinical guidelines from cardiology societies are also playing a role in harmonizing practices globally. This regional variation highlights a pressing need for investment in interventional training, cross-border collaborations, and policy support to ensure equitable access to life-saving cardiac interventions like BAV across all economic settings.How Is Innovation Shaping the Future of Balloon Aortic Valvuloplasty?
Innovative strides in device design, imaging modalities, and procedural workflows are rapidly transforming the landscape of Balloon Aortic Valvuloplasty. New-generation balloon catheters are characterized by lower profiles, enhanced trackability, and superior conformability to the native valve anatomy, which together reduce procedural trauma and facilitate more predictable outcomes. Integration with real-time 3D echocardiography and intraprocedural CT fusion imaging allows for precise valve positioning and real-time assessment of leaflet mobility and hemodynamic response. Innovations in anesthesia protocols and vascular access techniques, including the use of radial artery access, are also minimizing perioperative risks and reducing hospital stay durations. Some device manufacturers are even exploring biodegradable balloon technologies to further reduce procedural complications. Moreover, the development of combination devices that integrate valvuloplasty with drug delivery mechanisms or sensors for real-time pressure monitoring is poised to push the boundaries of therapeutic utility. Regulatory approvals for such novel devices are accelerating, particularly in Europe and the U.S., driven by fast-track designations for breakthrough cardiovascular technologies. Meanwhile, the digitization of procedural planning using AI-based anatomical simulations and patient-specific 3D-printed models is allowing cardiologists to anticipate outcomes with greater accuracy, improving procedural confidence. As a result, these innovations are not only expanding the applicability of BAV across diverse patient segments but are also laying the groundwork for its integration into precision cardiology.What Are the Key Catalysts Fueling Market Expansion Globally?
The growth in the Balloon Aortic Valvuloplasty market is driven by several factors rooted in clinical demand, technological advancements, and healthcare delivery trends. A primary driver is the rising prevalence of aortic stenosis globally, especially among the aging population, which has increased the number of high-risk patients unsuitable for immediate valve replacement. In parallel, the growing adoption of TAVR has led to greater use of BAV as a preparatory and evaluative tool, further expanding procedural volumes. Technologically, the availability of high-performance balloon catheters that cater to different anatomical and clinical needs has made the intervention safer and more effective. The increase in hybrid cardiac procedures and the evolving role of BAV in perioperative risk mitigation have also expanded its clinical indications. Additionally, shifting healthcare paradigms that emphasize shorter hospital stays and minimally invasive procedures are pushing providers toward less intensive interventions like BAV. From a consumer behavior standpoint, greater patient awareness and demand for low-risk, temporary solutions, especially in markets with long surgical wait times, are further influencing adoption. The emergence of outpatient cardiac care centers equipped with interventional capabilities is also making BAV more accessible. Moreover, favorable reimbursement frameworks in key regions such as North America and Europe are encouraging its use in both emergency and elective settings. Finally, increasing research and data supporting the efficacy of BAV in specific high-risk cohorts are prompting broader guideline inclusion, thus reinforcing its place in modern cardiac care strategies.Report Scope
The report analyzes the Balloon Aortic Valvuloplasty market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product Type (Non-Compliant Balloons, Semi-Compliant Balloons).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Non-Compliant Balloons segment, which is expected to reach US$100.1 Million by 2030 with a CAGR of a 2.8%. The Semi-Compliant Balloons segment is also set to grow at 4.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $34.8 Million in 2024, and China, forecasted to grow at an impressive 6.5% CAGR to reach $31.3 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Balloon Aortic Valvuloplasty Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Balloon Aortic Valvuloplasty Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Balloon Aortic Valvuloplasty Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as All-Clad, Anolon, Calphalon, Chicago Metallic, Circulon and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Balloon Aortic Valvuloplasty market report include:
- Abbott Laboratories
- B. Braun Melsungen AG
- Balt (Balt Extrusion)
- Balton Sp. z o. o.
- Becton Dickinson and Company
- Boston Scientific Corporation
- BrosMed Medical Co., Ltd.
- Cardinal Health, Inc.
- Cook Medical
- Edwards Lifesciences Corporation
- Getinge AB
- JOTEC GmbH
- Medtronic plc
- MicroPort Scientific Corporation
- NuMED, Inc.
- Philips (Koninklijke Philips N.V.)
- Stryker Corporation
- Terumo Corporation
- TT Medical, Inc.
- Venus MedTech (Hangzhou) Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- B. Braun Melsungen AG
- Balt (Balt Extrusion)
- Balton Sp. z o. o.
- Becton Dickinson and Company
- Boston Scientific Corporation
- BrosMed Medical Co., Ltd.
- Cardinal Health, Inc.
- Cook Medical
- Edwards Lifesciences Corporation
- Getinge AB
- JOTEC GmbH
- Medtronic plc
- MicroPort Scientific Corporation
- NuMED, Inc.
- Philips (Koninklijke Philips N.V.)
- Stryker Corporation
- Terumo Corporation
- TT Medical, Inc.
- Venus MedTech (Hangzhou) Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
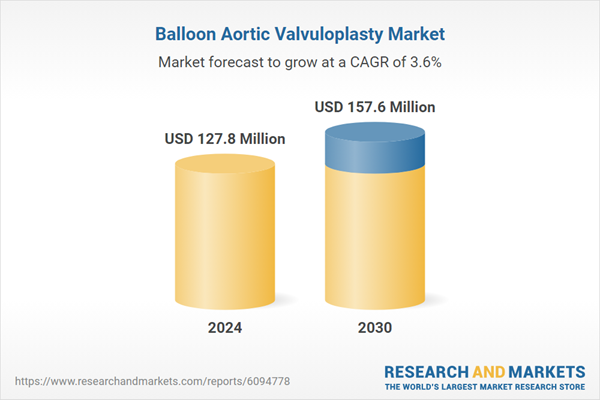
| Estimated Market Value ( USD | $ 127.8 Million |
| Forecasted Market Value ( USD | $ 157.6 Million |
| Compound Annual Growth Rate | 3.6% |
| Regions Covered | Global |