Global Cancer Active Pharmaceutical Ingredients (APIs) Market - Key Trends & Drivers Summarized
Why Are Cancer APIs Gaining Central Importance in the Global Pharmaceutical Industry?
Cancer Active Pharmaceutical Ingredients (APIs) represent one of the most critical and rapidly expanding segments of the global pharmaceutical landscape. As cancer remains a leading cause of morbidity and mortality worldwide, with millions of new cases diagnosed annually, the demand for effective, targeted, and advanced therapies has intensified dramatically. APIs are the biologically active components in oncology drugs that deliver therapeutic efficacy, and their production requires extreme precision, stringent regulatory oversight, and high-quality manufacturing processes. The rise in personalized medicine, fueled by breakthroughs in molecular biology and genomics, has heightened the need for APIs that can be tailored to specific cancer types, stages, and genetic markers. Unlike APIs for general-purpose medications, oncology APIs often involve highly potent compounds that require specialized containment, handling, and delivery systems. The increasing incidence of various cancers such as lung, breast, colorectal, and hematological malignancies has placed enormous emphasis on continuous innovation and scalability in API production. Moreover, with governments and healthcare organizations worldwide prioritizing cancer treatment within national healthcare agendas, investments in cancer drug development - both small molecule and biologic - are surging. APIs serve as the backbone of this development pipeline, making their reliable supply essential to public health. Pharmaceutical giants, contract manufacturing organizations (CMOs), and biotech firms are all ramping up capacity and compliance to meet the burgeoning global demand for high-potency APIs (HPAPIs). As oncology evolves into a dominant therapeutic area in drug development portfolios, cancer APIs are increasingly seen not just as chemical ingredients, but as strategic assets in the battle against one of the world's most formidable diseases.How Are Technology and Innovation Transforming the Development of Cancer APIs?
Technological innovation is reshaping every facet of cancer API development - from molecular discovery and synthesis to purification, scale-up, and quality control. One of the most significant advancements is the adoption of high-throughput screening and AI-powered drug discovery platforms, which are enabling faster identification of active molecules with anti-cancer properties. These tools accelerate early-stage research by simulating biological responses and identifying promising candidates that might otherwise be missed using traditional methods. In terms of synthesis, flow chemistry, and continuous manufacturing processes are revolutionizing production efficiency and safety, especially for HPAPIs that are hazardous to handle in batch operations. This shift allows for better containment, reduced waste, and greater control over critical process parameters. Analytical technologies like mass spectrometry, nuclear magnetic resonance (NMR), and real-time monitoring through PAT (Process Analytical Technology) are improving precision in purity profiling and structural verification, which is vital in oncology where even slight deviations can impact therapeutic outcomes. Additionally, the growing application of green chemistry principles is pushing manufacturers to adopt more sustainable and eco-friendly solvents, catalysts, and reaction pathways in API synthesis. On the biologics side, advancements in recombinant DNA technology and cell line engineering are improving yields and functionality of large-molecule APIs used in monoclonal antibodies, antibody-drug conjugates (ADCs), and CAR-T cell therapies. Regulatory technologies (RegTech) are also being deployed to streamline documentation, ensure traceability, and enhance GMP (Good Manufacturing Practice) compliance. These innovations are collectively enhancing the reliability, scalability, and regulatory alignment of cancer API manufacturing, ensuring the industry can keep pace with the complex demands of precision oncology.What Market Dynamics and Regulatory Factors Are Influencing the Growth of Cancer API Manufacturing?
The global market for cancer APIs is influenced by a combination of demand-side pressures, regulatory mandates, competitive dynamics, and geopolitical considerations. One major driver is the escalating incidence of cancer across aging populations and increasingly urbanized societies, which has led to rising global healthcare expenditures on oncology drugs. This demand is being met with a surge in research and development, particularly in regions like North America, Europe, and Asia-Pacific, where pharmaceutical and biotech firms are investing heavily in oncology pipelines. The role of Contract Development and Manufacturing Organizations (CDMOs) has grown significantly, as pharmaceutical companies look to outsource the complex, hazardous, and capital-intensive task of producing cancer APIs. Regulatory agencies such as the FDA (U.S.), EMA (Europe), PMDA (Japan), and CDSCO (India) are enforcing tighter scrutiny on API production facilities, with compliance to cGMP, ICH guidelines, and DMF (Drug Master File) submissions becoming mandatory for global market access. These regulatory frameworks influence not only quality assurance but also speed to market, particularly in the case of accelerated approval pathways for breakthrough cancer therapies. Meanwhile, the global push to reduce dependency on specific geographic regions - most notably China and India - for API sourcing has led to a diversification of supply chains, with governments incentivizing domestic production through policy reforms and infrastructure support. Intellectual property protections, pricing pressures, and generic competition also shape the cancer API landscape, especially for off-patent molecules where cost optimization and differentiation through manufacturing efficiency become key. These forces are collectively driving a more strategic, quality-focused, and regionally diversified cancer API market that is crucial to maintaining uninterrupted global oncology treatment supplies.What Strategic and Industry Trends Are Shaping the Future of the Cancer APIs Market?
The growth in the cancer active pharmaceutical ingredients market is driven by several strategic trends tied to personalization, specialization, and global manufacturing agility. One of the foremost trends is the rise of targeted therapy APIs, which are developed to interact with specific molecular targets involved in cancer progression, offering increased efficacy and fewer side effects compared to traditional cytotoxic agents. This shift is fostering demand for highly specific and structurally complex APIs that require advanced synthesis and purification capabilities. Another key driver is the increasing role of biologics and biosimilars in cancer treatment, necessitating large-scale capabilities in cell culture, fermentation, and protein engineering. Companies are investing in dual-platform production facilities capable of manufacturing both small-molecule and biologic APIs to serve diverse therapeutic portfolios. Strategic collaborations between pharmaceutical firms and CMOs/CDMOs are expanding, with a focus on co-development, tech transfer, and shared risk models to speed up commercialization timelines. Sustainability and ESG (Environmental, Social, and Governance) considerations are influencing investment decisions, with manufacturers adopting green chemistry, waste minimization strategies, and transparent sourcing to meet stakeholder expectations. Furthermore, digital transformation is enabling smarter manufacturing through data analytics, automation, and IoT-based monitoring, resulting in better batch consistency and regulatory compliance. The expansion of regulatory harmonization initiatives across regions is also easing market entry and lowering redundancy in documentation. Lastly, the increasing integration of companion diagnostics with cancer APIs is reinforcing the precision medicine paradigm, linking the success of a drug directly to a patient's genetic profile. These strategic shifts are ensuring that cancer APIs not only meet the growing volume demand but also evolve in sophistication, safety, and strategic alignment with the future of oncology care.Report Scope
The report analyzes the Cancer Active Pharmaceutical Ingredients (APIs) market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Innovative Type, Generic Type); Manufacturer (Captive Manufacturers, Merchant Manufacturers); Synthesis (Synthetic Oncology APIs, Biotech Oncology APIs); Indication (Lung Cancer Indication, Breast Cancer Indication, Colorectal Cancer Indication, Prostate Cancer Indication, Stomach Cancer Indication, Liver Cancer Indication, Other Indications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Innovative APIs segment, which is expected to reach US$38.4 Billion by 2030 with a CAGR of a 3.2%. The Generic APIs segment is also set to grow at 5.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $12.9 Billion in 2024, and China, forecasted to grow at an impressive 7% CAGR to reach $11.9 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Cancer Active Pharmaceutical Ingredients (APIs) Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Cancer Active Pharmaceutical Ingredients (APIs) Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Cancer Active Pharmaceutical Ingredients (APIs) Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as BLACK+DECKER, Bradshaw International, Cuisinart, Edlund Company, LLC, Ez-Duz-It and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 34 companies featured in this Cancer Active Pharmaceutical Ingredients (APIs) market report include:
- AbbVie Inc.
- Amgen Inc.
- Aurobindo Pharma Ltd.
- AstraZeneca plc
- Bayer AG
- Biocon Limited
- Bristol-Myers Squibb Company
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Eisai Co., Ltd.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Incyte Corporation
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Amgen Inc.
- Aurobindo Pharma Ltd.
- AstraZeneca plc
- Bayer AG
- Biocon Limited
- Bristol-Myers Squibb Company
- Cipla Ltd.
- Dr. Reddy's Laboratories Ltd.
- Eisai Co., Ltd.
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Incyte Corporation
- Johnson & Johnson
- Merck & Co., Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi S.A.
- Sun Pharmaceutical Industries Ltd.
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 466 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
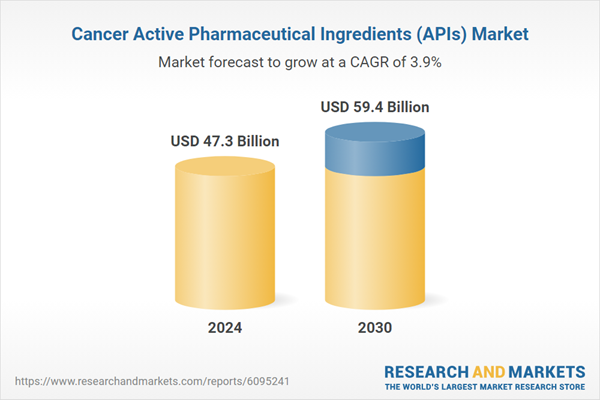
| Estimated Market Value ( USD | $ 47.3 Billion |
| Forecasted Market Value ( USD | $ 59.4 Billion |
| Compound Annual Growth Rate | 3.9% |
| Regions Covered | Global |