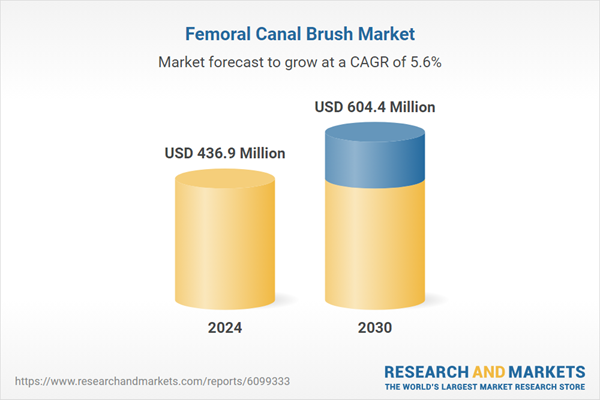
Global Femoral Canal Brush Market - Key Trends & Drivers Summarized
Why Is the Femoral Canal Brush Becoming a Crucial Surgical Tool in Orthopedics?
The femoral canal brush, a specialized surgical instrument used to clean and prepare the femoral canal before implant insertion in orthopedic procedures, is gaining significant traction in the global medical device market. Primarily used in total hip arthroplasty (THA) and revision surgeries, the brush plays a critical role in improving implant fixation by removing fat, marrow, and other debris that can compromise cement bonding or implant seating. As the number of hip replacement surgeries continues to rise globally - driven by aging populations, rising obesity rates, and higher incidences of osteoarthritis and trauma - the demand for instruments that enhance surgical outcomes and long-term implant stability is also increasing. The brush enables surgeons to achieve a cleaner and more consistent bone surface, reducing the risk of infection and aseptic loosening, two major causes of implant failure. Furthermore, as surgical protocols increasingly favor cemented fixation methods in elderly patients, the quality of canal preparation has come under greater scrutiny, making devices like femoral brushes indispensable in orthopedic practice. With growing emphasis on procedural efficiency, many manufacturers have responded by developing single-use, sterile-packed brushes that reduce intraoperative prep time and infection risk. Hospitals and surgical centers are also recognizing the brush's role in enhancing operative consistency and reproducibility, leading to its wider adoption in both academic and private healthcare institutions.How Are Material Innovation and Sterility Standards Transforming Brush Design?
Innovations in materials and manufacturing are significantly advancing the design, efficacy, and safety of femoral canal brushes. Traditional brushes composed of metal bristles or rigid plastics have evolved into more refined instruments using high-strength, flexible polymer bristles and ergonomic handles for improved surgeon control. These next-generation brushes are engineered to navigate the curvature and variable diameter of the femoral canal, ensuring comprehensive cleaning without damaging cancellous bone. The rise in single-use instrumentation, driven by infection control protocols and cost-efficiency in sterilization, has catalyzed the development of disposable femoral brushes that are pre-sterilized and designed for optimal one-time performance. In parallel, advancements in biocompatible materials ensure that bristle fragments or residual debris do not trigger inflammatory responses or interfere with implant fixation. Manufacturers are also innovating in handle design and brush tip geometry, offering surgeons greater tactile feedback and ease of use during minimally invasive approaches. Sterility assurance levels (SALs) have become a key differentiator, with regulatory bodies enforcing stringent validation processes for sterile-packaged devices. This has prompted OEMs to invest in high-integrity packaging, gamma sterilization techniques, and robust quality assurance protocols. Customization is another trend gaining ground, with brushes now offered in a range of diameters and lengths to match different implant sizes and surgical approaches. These design evolutions are not only making femoral brushes more reliable and surgeon-friendly but also aligning them with the broader trend of precision tools that support enhanced surgical outcomes in modern orthopedics.What Role Do Surgical Protocols and Training Play in Market Expansion?
The standardization of surgical protocols and the emphasis on surgeon training are critical to the rising adoption of femoral canal brushes in orthopedic operating rooms. Enhanced Recovery After Surgery (ERAS) programs and best-practice guidelines for hip arthroplasty now increasingly incorporate the use of femoral brushes as part of canal preparation, especially in cemented implant techniques. These protocols underscore the brush's importance in removing residual marrow elements and reducing cement interdigitation failure, which can otherwise lead to compromised mechanical stability. Surgical education platforms and orthopedic societies are actively promoting evidence-based instrumentation choices, and femoral canal brushes are frequently cited in literature as effective adjunct tools for achieving optimal bone bed conditions. As surgical training programs expand to include simulation labs and digital modules, hands-on familiarity with specialized instruments like femoral brushes is becoming a core component of orthopedic curricula. Surgeons trained in high-volume centers, where procedural consistency and outcomes tracking are emphasized, tend to adopt the latest tools that align with standardized workflows - driving broader demand. Additionally, regulatory agencies and hospital procurement departments are emphasizing tool traceability and performance benchmarking, further encouraging the inclusion of canal brushes in surgical kits. In revision surgeries and complex cases involving bone remodeling, the brush's utility becomes even more apparent, offering a quick and effective method for debriding compromised bone surfaces. As global health systems move toward value-based care and measurable outcomes, tools that improve implant longevity and reduce reoperation rates - like the femoral canal brush - are positioned for continued integration and growth in orthopedic practice.What Is Driving the Robust Growth of the Femoral Canal Brush Market Globally?
The growth in the global femoral canal brush market is driven by several factors closely linked to advancements in orthopedic procedures, aging demographics, healthcare infrastructure development, and shifting surgical preferences. First and foremost is the global rise in total hip replacement surgeries, fueled by an aging population, improved access to orthopedic care, and increasing incidence of musculoskeletal disorders. Countries across North America, Europe, and rapidly developing regions in Asia-Pacific are experiencing surges in hip arthroplasty procedures, boosting demand for adjunct surgical tools that ensure precision and reduce complication rates. A key technological driver is the shift toward cemented implant techniques in elderly patients, where optimal canal preparation using tools like the femoral brush is critical for long-term fixation. The growing preference for single-use surgical instruments in infection-prone environments is further accelerating demand, especially in high-volume hospitals and ambulatory surgical centers seeking operational efficiency. Additionally, the rise in orthopedic revision surgeries - due to implant loosening, infection, or wear - requires more meticulous canal decontamination, underscoring the brush's role in both primary and secondary procedures. Global expansion of orthopedic training programs and adoption of standardized surgical pathways are also pushing healthcare providers to invest in comprehensive surgical kits that include femoral brushes. Furthermore, regulatory approvals and product innovations from leading medical device manufacturers are enabling broader market penetration, with region-specific models tailored to anatomical and procedural differences. E-commerce and direct distribution channels are simplifying procurement for smaller hospitals and private practices, especially in emerging economies. These factors - anchored in surgical trends, demographic realities, and evolving healthcare standards - are collectively propelling the femoral canal brush from a niche instrument to a core component of modern orthopedic surgical practice.Report Scope
The report analyzes the Femoral Canal Brush market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Usage (Cleaning, Debride); Material (Disposable / Flexible Material, Twisted Material, Metallic Wire with Bristles Material); Application (Bone Cement Application, Hip Replacement Application, Joint Replacement Application, Other Applications); End-Use (Hospitals End-Use, Ambulatory Surgery Center End-Use, Orthopedic Clinics End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Cleaning Usage segment, which is expected to reach US$379 Million by 2030 with a CAGR of a 4.6%. The Debride Usage segment is also set to grow at 7.3% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $119 Million in 2024, and China, forecasted to grow at an impressive 8.6% CAGR to reach $120.5 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Femoral Canal Brush Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Femoral Canal Brush Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Femoral Canal Brush Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amagi, Amazon Freevee, Atmosphere TV, BBC Studios, Brightcove and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Femoral Canal Brush market report include:
- Arthrex, Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Biomet, Inc.
- ConMed Corporation
- DePuy Synthes
- Exactech, Inc.
- Geosurgical
- Medacta International
- Medline Industries, LP
- MicroPort Orthopedics Inc.
- Smith & Nephew plc
- Stryker Corporation
- Surgical Holdings
- United Orthopedic Corporation
- Waldemar Link GmbH & Co. KG
- Wright Medical Group N.V.
- Zimmer Biomet Holdings, Inc.
- Z-Medica, LLC
- ZSI Surgical Implants
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Arthrex, Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Biomet, Inc.
- ConMed Corporation
- DePuy Synthes
- Exactech, Inc.
- Geosurgical
- Medacta International
- Medline Industries, LP
- MicroPort Orthopedics Inc.
- Smith & Nephew plc
- Stryker Corporation
- Surgical Holdings
- United Orthopedic Corporation
- Waldemar Link GmbH & Co. KG
- Wright Medical Group N.V.
- Zimmer Biomet Holdings, Inc.
- Z-Medica, LLC
- ZSI Surgical Implants
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 468 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 436.9 Million |
| Forecasted Market Value ( USD | $ 604.4 Million |
| Compound Annual Growth Rate | 5.6% |
| Regions Covered | Global |