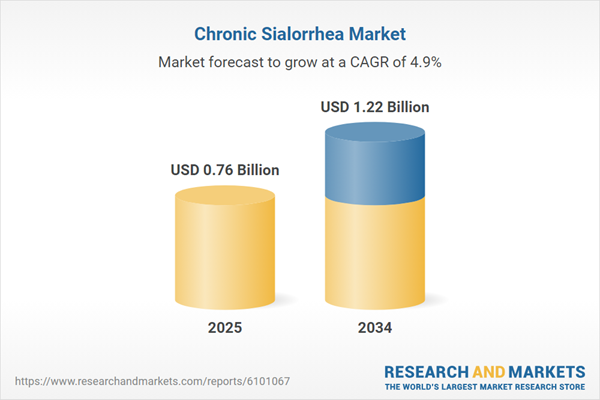
Chronic Sialorrhea Market Overview
Sialorrhea is a saliva-related medical condition that is also known as hypersalivation or excessive drooling. It is commonly of two types, namely, anterior sialorrhea and posterior sialorrhea. In anterior sialorrhea, excessive salivation is experienced by the patient running down from his/her mouth, leading to difficulty with cleanliness, skincare, and socialization. On the contrary, when patients are suffering from posterior sialorrhea, they experience excessive posterior spillage of saliva from their mouths down their airways (tracheas). This usually leads to chronic lung irritation.The market for chronic sialorrhea is impacted by the rising incidence of neurological conditions that stimulate this condition. Moreover, a surge in research and development initiatives to offer improved and efficient treatment to patients is one of the significant market trends. The key players in the market are involved in continuous mergers and collaborations to develop novel treatment alternatives that cater to a wide range of affected individuals.
Chronic Sialorrhea Market Growth Drivers
Regulatory Approvals Supporting Market Expansion
The market is experiencing an increased awareness about the serious repercussions of sialorrhea contributing to increased therapeutics developments, propelling market growth. The rising number of regulatory approvals by the authorities for drugs indicated for the treatment of chronic sialorrhea in children and adults is bolstering market growth. For instance, in November 2023, the Therapeutic Goods Administration (TGA) in Australia approved the use of XEOMIN by Merz Therapeutics for the treatment of children and adults suffering from chronic sialorrhea. This approval marked a significant milestone in the market as the XEOMIN is the first ever neurotoxin that has been approved by the regulatory authorities to treat the condition in Australia.Chronic Sialorrhea Market Trends
The market is witnessing several trends and developments to improve the current scenario. Some of the notable trends are as follows:Chronic Sialorrhea Market Segmentation
“Chronic Sialorrhea Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Breakup by Type
- Anterior Chronic Sialorrhea
- Posterior Chronic Sialorrhea
Market Breakup by Treatment
- Pharmacological Treatments
- Anticholinergics Glycopyrrolate Scopolamine Tropicamide Others
- Botulinum Toxin IncobotulinumtoxinA (Xeomin) RimabotulinumtoxinB (Myobloc)
- Invasive Treatments
- Surgery
- Radiotherapy
Market Breakup by Route of Administration
- Oral
- Sublingual
- Transdermal
- Intranasal
- Intramuscular
- Others
Market Breakup by Distribution Channel
- Retail Pharmacies
- Hospital Pharmacies
- Others
Market Breakup by Region
- United States
- EU-4 and the United Kingdom
- Japan
- India
Chronic Sialorrhea Market Share
Segmentation Based on Treatment Leads the Market Share
The market segmentation based on treatment is bifurcated into pharmacological treatments and invasive treatments. Pharmacological treatments are further bifurcated into anticholinergics and botulinum toxin including glycopyrrolate, scopolamine, and tropicamide, among others, and IncobotulinumtoxinA (Xeomin), and RimabotulinumtoxinB (Myobloc) respectively. The market share is expected to be led by pharmacological treatments due to their effectiveness and non-invasive experience. However, the treatment depends on the severity of the condition along with the patient profile. Therefore, invasive treatments which include surgery and radiotherapy are also expected to hold a notable market share in the forecast period, based on the symptoms of the patient.Chronic Sialorrhea Market Analysis by Region
Based on the region, the market includes the United States, EU-4 (Germany, France, Italy, Spain), and the United Kingdom, Japan, and India. The United States holds a significant share of the market majorly due to the growing geriatric population and rising prevalence of neurological conditions in the region. Neurological conditions such as Parkinson’s disease, and ALS, among others, are major conditions contributing to the occurrence of chronic sialorrhea in the region. The presence of robust healthcare infrastructure is also bolstering market growth as advanced technologies and medical facilities are highly reliable in the region.EU-4 which includes countries Germany, France, Italy, and Spain along with the United Kingdom are likely to witness significant market growth in the forecast period. The market growth can be attributed to the presence of key academic institutions which are engaged in advanced research to develop high-efficacy treatments for the condition.
Leading players in the Chronic Sialorrhea Market
The key features of the market report comprise patent analysis, clinical trial analysis, grants analysis and strategic initiatives by the leading players. The major companies in the market are as follows:US WorldMeds, LLC
Based in Louisville, Kentucky, it is an American speciality pharmaceutical company. The company specializes in developing licenses for unique healthcare products to support better patient outcomes and bridge the gap of unmet needs in the market. The company portfolio primarily focuses on neurology, oncology, and critical care.Merz Pharmaceuticals, LLC
Headquartered in Frankfurt, Germany, the company is a parent pharmaceutical company of independent businesses in several domains including aesthetic medicine, therapeutic medicine (neurological movement disorders included), and wellness and beauty products.Pfizer Inc
Headquartered in New York City, USA, the company is a multinational pharmaceutical and biotechnology corporation. The company specialises in developing and manufacturing medicines and vaccines for immunology, oncology, cardiology, endocrinology, and neurology.Ipsen Biopharmaceuticals, Inc
Based in Paris, France, it is a French biopharmaceutical company with a primary focus on drug development and commercialisation in oncology, rare disease, and neuroscience domains.Teva Pharmaceutical Industries Ltd
Based in Tel Aviv, Israel, the multinational pharmaceutical company focuses primarily on developing generic drugs. Additionally, the company is the largest manufacturer of generic drugs in the world.Other key players in the market include Boehringer Ingelheim International GmbH, GSK Plc, Bayer AG, McKesson Medical-Surgical Inc., Aurobindo Pharma Ltd, and Hikma Pharmaceuticals Plc
Key Questions Answered in the Chronic Sialorrhea Market
- What was the chronic sialorrhea market value in 2024?
- What is the chronic sialorrhea market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is market segmentation based on type?
- How is the market segmented based on treatment?
- What is market segmentation based on the route of administration?
- How is the market segmented based on distribution channels?
- Who are the end-users in the market?
- What are the major factors aiding the chronic sialorrhea market demand?
- How has the market performed so far and how is it anticipated to perform in the coming years?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major chronic sialorrhea market trends?
- Which route of administration is anticipated to witness significant market growth in the coming years?
- Which distribution channel is expected to dominate the market?
- Which type will dominate the market share?
- Which end-user is projected to contribute to the highest market growth?
- Who are the key players involved in the chronic sialorrhea market?
- What is the patent landscape of the market?
- How many clinical trials are being conducted for chronic sialorrhea?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- US WorldMeds, LLC
- Merz Pharmaceuticals, LLC
- Pfizer Inc.
- Ipsen Biopharmaceuticals, Inc.
- Teva Pharmaceutical Industries Ltd
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 0.76 Billion |
| Forecasted Market Value ( USD | $ 1.22 Billion |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |