Global Sialorrhea Treatment Market - Key Trends & Drivers Summarized
Why Is Sialorrhea Management Moving Beyond Symptomatic Relief?
Sialorrhea, or excessive drooling, has evolved from being perceived as a mere symptom of underlying neurological or developmental disorders to a target condition requiring focused clinical intervention. The complexity of its etiology-ranging from cerebral palsy and Parkinson-s disease to amyotrophic lateral sclerosis (ALS)-demands multifaceted treatment approaches. Historically, treatment revolved around supportive care such as absorbent products or behavioral therapy, but the shift toward addressing salivary gland function pharmacologically and surgically has expanded the clinical scope of intervention. The growing dissatisfaction with palliative care models has catalyzed investment in more definitive options, including botulinum toxin injections and surgical gland resection procedures, aiming to mitigate quality-of-life impairment, speech difficulties, and aspiration risks associated with chronic drooling.The transition from generalized neurology to targeted otolaryngological and interventional pharmacology strategies is redefining patient pathways. Clinicians are increasingly adopting structured diagnostic assessments, such as salivary flow quantification and videofluoroscopic swallowing studies, to inform treatment decisions. This shift underlines a broader trend: the prioritization of functional outcomes and precision in symptom control. Market dynamics are responding in parallel, with pharmaceutical companies and medical device manufacturers launching newer delivery systems-like guided ultrasound or MRI-assisted botulinum administration-to enhance efficacy and reduce procedural risks. As such, the treatment landscape for sialorrhea is transforming from symptom containment to root-function modulation, broadening opportunities across healthcare systems and technology developers alike.
Which Therapies Are Gaining Momentum Across Age and Condition Segments?
The segmentation of sialorrhea treatment modalities by age group and associated comorbidities is influencing product development and care protocols. In pediatric populations, particularly among children with cerebral palsy or genetic disorders, anticholinergic agents like glycopyrrolate are being utilized due to their non-invasive nature. However, side effect profiles such as constipation, drowsiness, or urinary retention limit long-term use. Consequently, intraglandular botulinum toxin injections have gained favor for their localized effect, reducing systemic exposure. These procedures are increasingly performed under ultrasound guidance to improve glandular targeting and minimize diffusion to surrounding musculature-a trend especially critical in pediatric care settings.In contrast, adult patients-especially those suffering from Parkinson-s disease or post-stroke conditions-require a different approach. Botulinum toxin type A (BoNT-A), specifically formulations like Xeomin® and Myobloc®, are witnessing increasing adoption due to their established safety profiles and repeatable efficacy in adult populations. Surgical interventions such as submandibular duct relocation or salivary gland excision are more commonly indicated in cases resistant to pharmacological treatment, particularly in institutionalized geriatric cohorts. These procedures are now being supported by image-guided techniques and shorter recovery times, making them more feasible even among high-risk patients. The rise in minimally invasive procedures also reflects the growing demand for solutions that balance long-term efficacy with lower post-procedural complication rates.
Moreover, individualized treatment algorithms are emerging as a key trend. Rather than standard protocols, clinicians are evaluating patients on the basis of drooling severity, cognitive function, swallowing safety, and underlying disease trajectory. This patient-centric approach is driving greater collaboration between neurology, otolaryngology, speech pathology, and pharmacology specialists. As multidisciplinary treatment models gain ground, demand for integrated digital platforms and clinical decision-support tools is rising, opening new avenues for software developers and remote care providers.
How Is Technology Transforming Delivery and Monitoring in Sialorrhea Care?
Technology is now at the forefront of sialorrhea management, enhancing both therapeutic efficacy and clinical oversight. One of the most notable developments is the integration of imaging tools-particularly high-resolution ultrasound and MRI-for guiding botulinum toxin injections. This has enabled more precise delivery into the parotid and submandibular glands while significantly reducing procedural variability. In clinical trials and real-world practice, image-guided injections have demonstrated improved response duration and a lower incidence of side effects, such as dysphagia or xerostomia, positioning them as a standard-of-care advancement.Additionally, wearable devices and app-based drooling diaries are facilitating more accurate symptom tracking, particularly in pediatric patients or those with cognitive decline. Caregivers and clinicians are using these digital tools to monitor treatment outcomes over time, assess changes in salivary volume, and tailor medication dosages or intervention frequency. This real-time data acquisition supports more responsive clinical decisions and enhances adherence monitoring, an area where traditional paper-based assessments were often inadequate. These platforms are also enabling remote monitoring in patients residing in long-term care facilities or under telehealth supervision, expanding the reach of specialist care.
Further, the use of AI-based diagnostic aids and decision-support algorithms is showing promise in stratifying patients for different treatment modalities. Systems that analyze patient history, neurological assessment scores, and prior treatment response are being tested to recommend personalized therapy plans. Meanwhile, pharmaceutical delivery technologies such as sustained-release buccal films and transdermal patches for anticholinergics are under development to bypass gastrointestinal absorption and minimize systemic side effects. As these innovations enter clinical practice, they are poised to disrupt conventional prescription pathways and stimulate competition in specialty pharmaceutical markets.
What Are the Market Forces Accelerating Global Adoption of Sialorrhea Solutions?
The growth in the global sialorrhea treatment market is driven by several factors that converge around rising disease prevalence, unmet clinical needs, and the emergence of precision-based care models. A surge in neurodegenerative disorders-especially Parkinson-s disease and ALS-across aging populations in North America, Europe, and parts of Asia-Pacific is catalyzing demand for drooling management therapies. Simultaneously, increasing survival rates among pediatric neurological patients have created a chronic care segment that requires long-term drooling mitigation strategies. This dual demographic pressure is prompting healthcare systems and payers to invest in cost-effective, scalable solutions, such as outpatient botulinum clinics and mobile health monitoring.Another major driver is regulatory support for novel therapeutics. Approvals of drugs like glycopyrrolate in oral solution form (e.g., Cuvposa®) and continued FDA fast-track reviews for reformulated anticholinergics have spurred innovation. These approvals reduce time-to-market and encourage investment in next-generation compounds with improved safety and tolerability. Reimbursement frameworks, particularly in publicly funded healthcare markets, are evolving to include botulinum toxin procedures for sialorrhea, increasing accessibility and boosting procedure volumes. Private insurers are also expanding coverage due to rising evidence of cost savings related to reduced hospitalizations for aspiration pneumonia or speech therapy sessions.
Market expansion is further supported by strategic partnerships between pharma and device companies aiming to deliver combined product-service ecosystems. Botulinum toxin producers are collaborating with imaging device manufacturers and telehealth platforms to bundle therapeutic products with diagnostic and follow-up tools. This integrated approach not only boosts patient outcomes but also supports value-based reimbursement models gaining traction globally. Moreover, the entrance of regional players into emerging markets in Latin America, Eastern Europe, and Southeast Asia is localizing production and distribution, reducing dependence on high-cost imports and enhancing market penetration.
In conclusion, the sialorrhea treatment market is witnessing a dynamic shift from generalized symptom control to tailored, multidisciplinary management enabled by cutting-edge technology and evolving clinical frameworks. These developments are expected to accelerate innovation pipelines and broaden access, transforming patient experiences across diverse age groups and neurological conditions.
Scope Of Study:
The report analyzes the Sialorrhea Treatment market in terms of units by the following Segments, and Geographic Regions/Countries:Segments: Medical Therapy (Pharmacologic Therapy, Radiotherapy); End-Use (Hospitals End-Use, Specialty Clinics End-Use, Homecare Settings End-Use)
Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Pharmacologic Therapy segment, which is expected to reach US$607.6 Million by 2030 with a CAGR of a 4.9%. The Radiotherapy segment is also set to grow at 2.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, estimated at $203.3 Million in 2024, and China, forecasted to grow at an impressive 7.6% CAGR to reach $194.0 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Sialorrhea Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Sialorrhea Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Sialorrhea Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aurobindo Pharma Limited, Bayer AG, Boehringer Ingelheim International GmbH, Bristol Myers Squibb Company, Clini Experts Services Pvt Ltd and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Sialorrhea Treatment market report include:
- Aurobindo Pharma Limited
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Clini Experts Services Pvt Ltd
- Eisai Co. Ltd.
- ExCEEd Orphan Pharmaceuticals AG
- Fresenius Kabi AG
- Gufic Biosciences Limited
- Hikma Pharmaceuticals PLC
- Ipsen Pharmaceuticals Inc.
- Merz Therapeutics GmbH
- Medy-Tox Inc.
- Neos Therapeutics Inc.
- NeuroHealing Pharmaceuticals Inc.
- Pfizer Inc.
- Proveca Limited
- Shionogi Inc.
- Solstice Neurosciences Inc.
- Supernus Pharmaceuticals Inc.
- Taj Pharma India Limited
- US WorldMeds LLC
This edition integrates the latest global trade and economic shifts as of June 2025 into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes segmentation by product, technology, type, material, distribution channel, application, and end-use, with historical analysis since 2015.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
- Complimentary Update: Buyers receive a free July 2025 update with finalized tariff impacts, new trade agreement effects, revised projections, and expanded country-level coverage.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aurobindo Pharma Limited
- Bayer AG
- Boehringer Ingelheim International GmbH
- Bristol Myers Squibb Company
- Clini Experts Services Pvt Ltd
- Eisai Co. Ltd.
- ExCEEd Orphan Pharmaceuticals AG
- Fresenius Kabi AG
- Gufic Biosciences Limited
- Hikma Pharmaceuticals PLC
- Ipsen Pharmaceuticals Inc.
- Merz Therapeutics GmbH
- Medy-Tox Inc.
- Neos Therapeutics Inc.
- NeuroHealing Pharmaceuticals Inc.
- Pfizer Inc.
- Proveca Limited
- Shionogi Inc.
- Solstice Neurosciences Inc.
- Supernus Pharmaceuticals Inc.
- Taj Pharma India Limited
- US WorldMeds LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 278 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
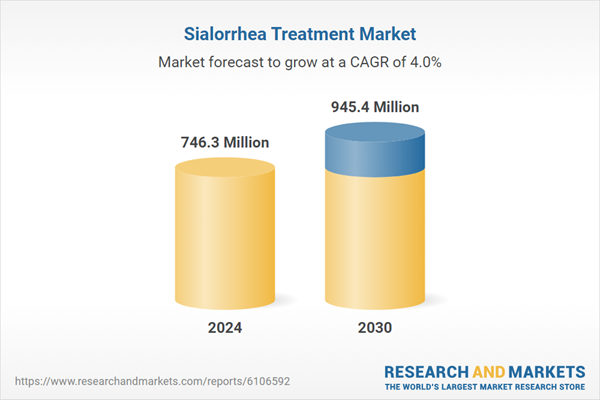
| Estimated Market Value in 2024 | 746.3 Million |
| Forecasted Market Value by 2030 | 945.4 Million |
| Compound Annual Growth Rate | 4.0% |
| Regions Covered | Global |