Viral Clearance Services Market Overview
Viral clearance services ensure the complete removal or inactivation of viruses in biopharmaceutical production, thus assuring the safety and efficacy of the product. Key drivers of growth of the market are increasing demand for biologics, gene therapy, and rising incidences of chronic diseases. Several trends such as regulatory compliance, biotechnology innovations, and strategic partnerships continue to propel the market growth. The market holds significant growth potential, fueled by advancements in biotechnology and rising investments in innovative next-generation therapies.Viral Clearance Services Market Growth Drivers
Rising Prevalence of Chronic Disease Boost the Market Demand
The growing prevalence of chronic diseases has become a significant driver of market expansion. Conditions such as cancer, diabetes, and autoimmune disorders require the implementation of advanced biologics and gene therapies, which are governed by stringent viral safety protocols to comply with regulatory standards and protect patient health. The growing burden of these chronic diseases raises the demand for new therapeutics alongside the need for effective viral clearance procedures to mitigate the contamination risks during manufacturing.Viral Clearance Services Market Trends
Several trends and developments are being observed in the market to enhance the current situation. Some of the noteworthy trends are as follows:Growing Biopharmaceutical Demand to Boost Market Value
The market is witnessing a notable surge in outsourcing biopharmaceuticals, reflecting a strategic shift towards efficiency, cost-effectiveness, and specialized expertise. As per the analysis by Expert Market Research, the global biopharmaceutical market is expected to grow at a CAGR of 7.6% in the forecast period of 2025-2034. This growth is further fueled by factors such as advancements in biotechnology, increasing prevalence of chronic diseases, and rising investments in research and development.Exclusive Partnerships to Strengthen the Viral Clearance Service Market Size
In December 2023, an exclusive partnership between Summit Pharmaceuticals International Corporation (SPI) and Texcell Japan marked a pivotal development in the market. The collaboration was intended to enhance SPI’s capabilities in developing antibody, exosome, and nucleic acid therapeutics with Texcell’s esteemed reputation in GMP/GLP-certified viral clearance services and over 35 years of expertise in viral safety testing. This partnership exemplifies a strategic move to foster innovative treatment solutions while reinforcing the safety framework essential for the growth of the pharmaceutical landscape.Regulatory Compliance Boosting the Viral Clearance Service Market Value
The market is being significantly shaped by innovations that comply with stringent regulatory standards. For instance, in February 2024, Merck launched the Deviron™ detergent portfolio to exceed traditional virus inactivation methodologies. This development allows effective virus inactivation to mitigate contamination risks in pharmaceutical products.Rise in Investments to Drive Viral Clearance Service Market Growth
Strategic investments are pivotal in catalyzing growth within the market, as it is increasingly essential for ensuring the safety and efficacy of biopharmaceutical products. For instance, in June 2023, the National Institute for Innovation in Manufacturing Biopharmaceuticals (NIIMBL) awarded funding of USD 15.8 million to Cytiva and BioCentriq. This investment aimed to enhance the development of a viral and exotoxin clearance platform specifically tailored for adeno-associated virus (AAV) manufacturing processes. Such financial backing underscores the significance of innovative technologies in addressing the complexities of viral clearance, thereby fostering market advancement. As the demand for more effective biomanufacturing processes grows, strategic investments will play a crucial role in propelling the viral clearance service market in the forecast period.Viral Clearance Services Market Segmentation
The market report offers a detailed analysis of the market based on the following segments:
Market Breakup by Method
- Viral Removal Method
- Viral Inactivation Method
- Chemical
- Radiation
- Others
Market Breakup by Application
- Recombinant Proteins
- Tissue and Blood-derived Products
- Vaccines
- Others
Market Breakup by End User
- Biopharmaceuticals
- Contract Research Organizations
- Academic Research Institutes
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Viral Clearance Services Market Share
Market Share by Method to Witness Significant Growth
Based on method, the market is divided into viral removal method, viral inactivation method, and others. Among these, the viral removal method is expected to dominate the market due to its superior efficacy in the physical elimination of viruses from biotherapeutics, thereby ensuring product safety. This technique has gained prominence within the biopharmaceutical sector, driven by advancements in filtration and chromatography technologies. The viral removal approach not only meets evolving safety regulations but also offers innovative solutions that will likely facilitate market expansion during the forecast period.Viral Clearance Services Market Analysis by Region
Regionally, the market is divided into North America, Europe, Asia Pacific, Latin America, Middle East and Africa. Among these, North America is expected to dominate the market due to its well-established biopharmaceutical infrastructure. For instance, in June 2023, Texcell announced its new 27,000-square-foot viral clearance testing facility in Frederick, Maryland. This facility exemplifies advanced infrastructure dedicated to improving viral safety protocols for monoclonal antibodies, gene therapies, and medical devices. Designed with a modular layout, it provides the flexibility required for simultaneous viral clearance and biosafety studies, addressing the evolving demands of the contract testing landscape. Such initiatives reinforce regulatory compliance, ultimately contributing to the overall growth and resilience of the market.Leading Players in the Viral Clearance Services Market
The key features of the market report include grants analysis, funding and investment analysis, and strategic initiatives by the leading players. The major companies are:Charles River Laboratories International, Inc
Established in 1947 and headquartered in Wilmington, Massachusetts, Charles River Laboratories is a global leader in drug development services. Its viral clearance product portfolio includes comprehensive validation studies to ensure the safety and efficacy of biopharmaceutical products. Key offerings include virus filtration, inactivation, and clearance testing services, which are part of their biologics testing solutions.Labor Dr. Merk & Kollegen GmbH
Founded in 1971 and based in Ochsenhausen, Germany, Labor Dr. Merk & Kollegen GmbH specializes in GLP and GMP-certified biosafety testing. Their expertise includes viral clearance services for viral vectors, oncolytic viruses, and biopharmaceutical products. The company offers tailored solutions for virus inactivation and filtration, ensuring compliance with international regulatory standards. Their reputation lies in supporting the development of innovative therapeutic solutions with a strong focus on viral safety.Merck KGaA
Established in 1668 and headquartered in Darmstadt, Germany, Merck KGaA is a pioneer in life sciences and biopharmaceutical services. Through its BioReliance® portfolio, Merck provides viral clearance testing, including virus filtration and inactivation services. Their innovative solutions, such as the recently launched Deviron™ detergents, cater to evolving regulatory demands and biopharmaceutical manufacturing needs, emphasizing compliance, safety, and efficiency in viral clearance processes.Texcell SA
Founded in 1987 and headquartered in France, Texcell SA is a global leader in viral safety testing and viral clearance studies. Their product portfolio includes biosafety testing for monoclonal antibodies, gene therapies, and vaccines. Texcell’s expertise spans over 35 years, offering robust viral inactivation and filtration services. The company is renowned for supporting the development and regulatory compliance of biopharmaceuticals and medical devices worldwide.Other key players in the market include WuXi AppTec Co., Ltd, Sartorius AG, Eurofins Scientific, Syngene International Limited, and Lonza AG.
Key Questions Answered in the Global Viral Clearance Services Market
- What was the global viral clearance services market value in 2024?
- What is the global viral clearance services market forecast outlook for 2025-2034?
- What are the regional markets covered in the report?
- What is the market segmentation based on the method?
- What is the market breakup based on the application?
- What is the market segmentation based on the end user?
- What are the major factors aiding the viral clearance services market demand?
- What are the market's major drivers, opportunities, and restraints?
- Which regional market is expected to lead the market share in the forecast period?
- Which country is expected to experience expedited growth during the forecast period?
- What are the major viral clearance services market trends?
- How does the rise in the geriatric population impact the market size?
- Who are the key players involved in the viral clearance services market?
- What are the current unmet needs and challenges in the market?
- How are partnerships, collaborations, mergers, and acquisitions among the key market players shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Charles River Laboratories International, Inc.
- Labor Dr. Merk & Kollegen GmbH
- Merck KGaA
- Texcell SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
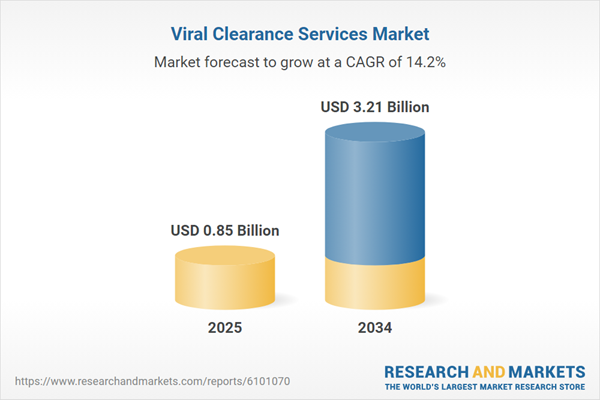
| Estimated Market Value ( USD | $ 0.85 Billion |
| Forecasted Market Value ( USD | $ 3.21 Billion |
| Compound Annual Growth Rate | 14.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 4 |