Global Viral Clearance Market - Key Trends & Drivers Summarized
Why Is Viral Clearance Critical for Biopharmaceutical Safety?
Viral clearance has become an essential component in the development and manufacturing of biologics, ensuring the removal of potential viral contaminants from biopharmaceutical products. The rise in monoclonal antibodies, recombinant proteins, and gene therapy products has significantly increased the focus on stringent viral safety protocols. Regulatory agencies such as the FDA and EMA mandate comprehensive viral clearance studies to validate manufacturing processes, driving demand for advanced viral inactivation and removal technologies. Biopharmaceutical companies are increasingly investing in viral clearance solutions, including filtration, chromatography, and chemical inactivation, to meet these regulatory requirements and ensure product safety.How Are Technological Innovations Transforming Viral Clearance Methods?
The market is witnessing a surge in technological innovations aimed at improving the efficiency and reliability of viral clearance. Advances in high-throughput screening, automation, and AI-driven analytics are streamlining viral clearance processes, reducing turnaround times, and enhancing accuracy. Novel filtration techniques, including nanofiltration and membrane chromatography, are gaining traction for their superior viral removal capabilities. The growing adoption of single-use bioprocessing systems has also necessitated the development of compatible viral clearance solutions, ensuring seamless integration into modern manufacturing workflows. Additionally, the increasing role of continuous bioprocessing is driving the need for real-time viral clearance monitoring and validation technologies.What Market Dynamics Are Influencing the Growth of Viral Clearance Services?
The demand for viral clearance services is expanding as pharmaceutical companies increasingly outsource biologics manufacturing to contract research organizations (CROs) and contract development and manufacturing organizations (CDMOs). The growing complexity of biologic drugs, coupled with stringent regulatory oversight, has led to an increase in specialized viral clearance testing services. The emergence of new biotherapeutics, including cell and gene therapies, has further intensified the need for reliable and scalable viral safety solutions. Additionally, the expanding vaccine development landscape, particularly in response to global health crises, is reinforcing the necessity of robust viral clearance strategies to ensure the safety and efficacy of immunotherapies.The growth in the viral clearance market is driven by several factors, including the increasing number of biologics in the drug pipeline, advancements in viral removal technologies, and the rising outsourcing of biopharmaceutical manufacturing. The surge in gene and cell therapy development is further accelerating demand for comprehensive viral safety solutions. Additionally, the integration of AI and machine learning in viral clearance testing is enhancing process efficiency and regulatory compliance. The increasing adoption of single-use and continuous bioprocessing systems is also influencing market dynamics, necessitating innovative viral inactivation and filtration techniques. As regulatory standards continue to evolve, biopharmaceutical companies are investing in state-of-the-art viral clearance platforms to ensure product safety and expedite market approvals, driving sustained market growth.
Report Scope
The report analyzes the Viral Clearance market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Application (Recombinant Proteins, Blood and Blood Products, Vaccines, Other Applications); End-Use (Pharmaceutical & Biotechnology, CROs, Academic Research Institutes, Other End-Uses).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Recombinant Proteins Application segment, which is expected to reach US$467.7 Million by 2030 with a CAGR of a 18.5%. The Blood and Blood Products Application segment is also set to grow at 15.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $215.9 Million in 2024, and China, forecasted to grow at an impressive 17.8% CAGR to reach $319.2 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Viral Clearance Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Viral Clearance Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Viral Clearance Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Avance Biosciences Inc., BSL BIOSERVICE Scientific Laboratories Munich GmbH, Charles River Laboratories International, Inc., Clean Cells Inc., Lonza Group AG and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 41 companies featured in this Viral Clearance market report include:
- Avance Biosciences Inc.
- BSL BIOSERVICE Scientific Laboratories Munich GmbH
- Charles River Laboratories International, Inc.
- Clean Cells Inc.
- Lonza Group AG
- Merck KgaA
- SGS SA
- Sigma-Aldrich Corporation
- Texcell, Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Avance Biosciences Inc.
- BSL BIOSERVICE Scientific Laboratories Munich GmbH
- Charles River Laboratories International, Inc.
- Clean Cells Inc.
- Lonza Group AG
- Merck KgaA
- SGS SA
- Sigma-Aldrich Corporation
- Texcell, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
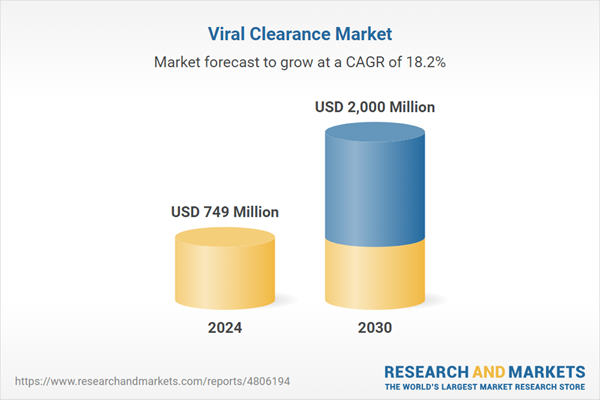
| Estimated Market Value ( USD | $ 749 Million |
| Forecasted Market Value ( USD | $ 2000 Million |
| Compound Annual Growth Rate | 18.2% |
| Regions Covered | Global |