Global Lysosomal Disease Treatment Market - Key Trends & Drivers Summarized
Why Is Lysosomal Disease Treatment Gaining Increasing Importance in Modern Therapeutics?
Lysosomal diseases, also known as lysosomal storage disorders (LSDs), are a group of rare genetic conditions that result from the malfunction or absence of specific enzymes responsible for breaking down various substances in the body's cells. The accumulation of these substances within the lysosomes leads to progressive cellular damage, impacting vital organs and systems. Historically, the diagnosis and treatment of LSDs were limited, with patients facing a life marked by significant disability and reduced lifespan. However, recent advancements in diagnostics, biotechnology, and therapeutic development have begun to transform the outlook for individuals with these complex conditions. Lysosomal disease treatment is now gaining traction within the rare disease research ecosystem, reflecting a broader shift toward precision medicine and patient-specific care. Increasing public awareness, advocacy from patient groups, and orphan drug incentives have contributed to greater focus and investment in this space. As a result, LSDs have transitioned from being largely untreatable to being among the most actively researched rare disorders in the pharmaceutical landscape. Conditions such as Gaucher disease, Fabry disease, Pompe disease, and various forms of mucopolysaccharidoses (MPS) are now at the forefront of genetic and enzyme replacement research. The urgency to address these life-threatening diseases, combined with the rapid pace of innovation in gene therapy and biologics, is positioning lysosomal disease treatment as a key frontier in the advancement of modern rare disease therapeutics.How Are Scientific Advances and Treatment Modalities Evolving in Lysosomal Disease Therapy?
The treatment landscape for lysosomal diseases is evolving rapidly, fueled by groundbreaking developments in enzyme replacement therapy (ERT), substrate reduction therapy (SRT), gene therapy, and pharmacological chaperones. ERT has been the most established approach, involving the intravenous infusion of synthetic versions of the deficient enzymes to restore lysosomal function. While effective in alleviating certain symptoms and improving quality of life, ERT often struggles to address neurological manifestations due to its inability to cross the blood-brain barrier. This limitation has spurred innovation in gene therapy, where therapeutic genes are delivered directly into patients' cells using viral vectors such as AAV (adeno-associated virus). These gene therapies aim to provide a long-term solution by enabling the patient's own cells to produce the necessary enzymes, offering hope for systemic and central nervous system involvement. Pharmacological chaperones are another emerging option, working by stabilizing the misfolded enzymes and enhancing their function. Meanwhile, substrate reduction therapies aim to decrease the build-up of toxic substrates by limiting their production, offering a complementary strategy to enzyme-focused approaches. Advances in CRISPR gene editing, exosome-based delivery, and nanomedicine are also under investigation as potential future tools to target lysosomal dysfunction more precisely. Importantly, personalized treatment regimens are becoming more viable due to improved diagnostic capabilities, such as next-generation sequencing and biomarker identification, which allow early and accurate identification of LSD subtypes. These evolving treatment modalities are expanding the clinical toolkit available to physicians and researchers, increasing both life expectancy and quality of life for patients with lysosomal storage disorders.What Market Trends and Healthcare Developments Are Shaping the Lysosomal Disease Treatment Ecosystem?
The lysosomal disease treatment ecosystem is being shaped by a complex interplay of market trends, regulatory dynamics, healthcare policies, and technological developments. A significant driver of growth is the increasing global focus on rare and orphan diseases, which is drawing attention to previously underserved patient populations. Regulatory agencies such as the FDA and EMA are offering incentives like fast-track designations, orphan drug status, and extended exclusivity periods to encourage innovation in this area. As a result, biopharmaceutical companies are investing heavily in lysosomal disease pipelines, leading to a growing number of clinical trials and new therapeutic approvals. Advances in newborn screening programs and genomic sequencing are enabling earlier detection and intervention, which is crucial for managing progressive disorders like LSDs. Healthcare systems are also adapting by developing specialized centers of excellence and multidisciplinary care teams to address the multifaceted needs of LSD patients. Additionally, there is a growing recognition of the burden these diseases place on families and caregivers, prompting the inclusion of quality-of-life metrics and caregiver support in treatment plans. The rise of digital health tools, including remote monitoring and patient registries, is enhancing disease tracking and data collection, contributing to better long-term outcomes. Moreover, increasing global collaboration among researchers, clinicians, and advocacy organizations is accelerating knowledge exchange and harmonizing treatment guidelines. These shifts are not only improving clinical care but are also making the overall lysosomal disease treatment market more dynamic, inclusive, and patient-centered, creating a foundation for sustained innovation and global accessibility.What Are the Core Drivers Fueling the Growth of the Global Lysosomal Disease Treatment Market?
The growth in the global lysosomal disease treatment market is driven by several interrelated factors rooted in scientific progress, healthcare infrastructure development, evolving regulatory frameworks, and changing stakeholder priorities. A primary driver is the expanding prevalence data due to improved diagnostic capabilities, particularly through genetic testing and newborn screening programs, which are identifying patients earlier and more accurately. The surge in investment by biotechnology and pharmaceutical firms into rare disease portfolios is another key contributor, with lysosomal storage disorders becoming a focal point for cutting-edge therapeutic research, including gene editing and enzyme engineering. Favorable regulatory policies, including orphan drug designations, accelerated approval pathways, and reimbursement support in developed markets, are encouraging commercial innovation and reducing market-entry barriers for new therapies. The rise of patient advocacy and global awareness campaigns is also influencing healthcare policy and encouraging collaboration among stakeholders, pushing for faster access to life-saving treatments. Technological advances in drug delivery systems, biomarker discovery, and bioinformatics are facilitating more effective and personalized treatment strategies. Strategic partnerships between academia and industry are driving translational research from bench to bedside, while healthcare providers are increasingly creating integrated care pathways that include genetic counseling, psychosocial support, and long-term monitoring. Furthermore, the demand for therapies that address unmet neurological and systemic needs in lysosomal diseases is prompting the development of next-generation platforms that go beyond symptom management to target the root causes of disease. These combined drivers are ensuring that lysosomal disease treatment not only remains a high-impact therapeutic focus but also represents one of the most dynamic and promising areas within the rare disease and precision medicine landscape.Report Scope
The report analyzes the Lysosomal Disease Treatment market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Disease Type (Mucopolysaccharidosis Disease, Pompes Syndrome Disease, Fabry Diseases, Gaucher's Disease, Other Disease Types); Therapy (Substrate Reduction Therapy, Stem Cell Therapy, Enzyme Replacement Therapy, Other Therapies); Administration Route (Oral Administration, Parenteral Administration, Other Administration Routes); End-Use (Hospitals End-Use, Specialty Clinics End-Use, Homecare End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Mucopolysaccharidosis Disease segment, which is expected to reach US$4.4 Billion by 2030 with a CAGR of a 6.9%. The Pompes Syndrome Disease segment is also set to grow at 4.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.2 Billion in 2024, and China, forecasted to grow at an impressive 8.8% CAGR to reach $2.3 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Lysosomal Disease Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Lysosomal Disease Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Lysosomal Disease Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as A.?Lange?&?Söhne, Audemars?Piguet, Breguet, Breitling, Cartier and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Lysosomal Disease Treatment market report include:
- Alexion Pharmaceuticals (AstraZeneca)
- Amicus Therapeutics, Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- BioMarin Pharmaceutical Inc.
- Chiesi Farmaceutici S.p.A.
- Eli Lilly and Company
- Horizon Therapeutics plc
- Johnson & Johnson Services Inc.
- Merck & Co., Inc.
- Novartis International AG
- Orphazyme A/S
- Pfizer Inc.
- Protalix BioTherapeutics, Inc.
- Raptor Pharmaceuticals
- Recordati Industria Chimica S.p.A.
- Sanofi (Genzyme)
- Sigilon Therapeutics, Inc.
- Shire (Takeda)
- Ultragenyx Pharmaceutical Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alexion Pharmaceuticals (AstraZeneca)
- Amicus Therapeutics, Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- BioMarin Pharmaceutical Inc.
- Chiesi Farmaceutici S.p.A.
- Eli Lilly and Company
- Horizon Therapeutics plc
- Johnson & Johnson Services Inc.
- Merck & Co., Inc.
- Novartis International AG
- Orphazyme A/S
- Pfizer Inc.
- Protalix BioTherapeutics, Inc.
- Raptor Pharmaceuticals
- Recordati Industria Chimica S.p.A.
- Sanofi (Genzyme)
- Sigilon Therapeutics, Inc.
- Shire (Takeda)
- Ultragenyx Pharmaceutical Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 477 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
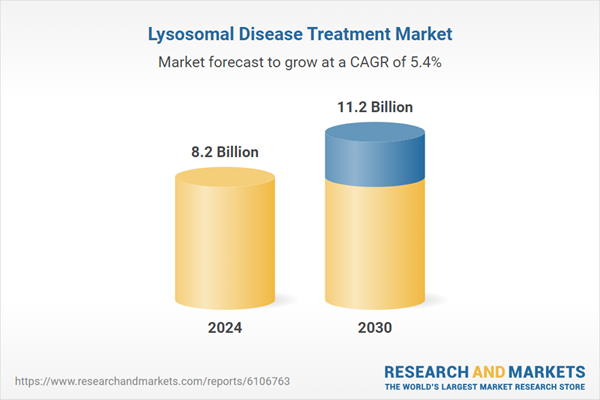
| Estimated Market Value ( USD | $ 8.2 Billion |
| Forecasted Market Value ( USD | $ 11.2 Billion |
| Compound Annual Growth Rate | 5.4% |
| Regions Covered | Global |