Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Rising Prevalence of Chronic and Autoimmune Diseases
The increasing incidence of chronic and autoimmune diseases is a key factor driving growth in the Global Biosimilar Testing Services Market, as it boosts demand for affordable biologic therapies. Chronic noncommunicable diseases (NCDs) continue to be a major global health concern. According to the World Health Organization (WHO), NCDs were responsible for approximately 43 million deaths in 2021 - about 75% of all non-pandemic-related fatalities. Cardiovascular diseases led with 19 million deaths, followed by cancers (10 million), chronic respiratory illnesses (4 million), and diabetes-related complications (over 2 million). The high burden of these diseases has intensified the need for biosimilars as cost-effective alternatives, thereby increasing the demand for comprehensive testing services to ensure therapeutic equivalence and safety.Key Market Challenges
High Complexity and Cost of Biosimilar Development and Testing
The intricate nature and high cost of biosimilar development and testing remain major challenges in the Global Biosimilar Testing Services Market. Unlike small-molecule generics, biosimilars are complex biologics produced using living systems, requiring advanced analytical and functional characterization to establish similarity with reference products.Technologies such as mass spectrometry, chromatography, capillary electrophoresis, and NMR spectroscopy are essential for detailed analysis, significantly raising development expenses. Biosimilar developers must also conduct robust comparability assessments, potency tests, immunogenicity evaluations, pharmacokinetic/pharmacodynamic studies, and clinical trials to comply with rigorous regulations set by bodies like the U.S. FDA, EMA, and PMDA. These multifaceted regulatory demands lead to prolonged development timelines and substantial financial investment, posing barriers especially for small and mid-sized firms.
Key Market Trends
Increasing Adoption of Advanced Analytical Techniques for Biosimilar Characterization
A prominent trend in the Global Biosimilar Testing Services Market is the growing reliance on advanced analytical tools for biosimilar characterization. To meet regulatory expectations set by agencies such as the U.S. FDA, EMA, and PMDA, biosimilar developers must demonstrate structural and functional equivalence to originator biologics. Traditional methods often lack the sensitivity to detect subtle molecular differences, prompting increased use of high-resolution mass spectrometry (HRMS), nuclear magnetic resonance (NMR) spectroscopy, capillary electrophoresis (CE), and surface plasmon resonance (SPR). These technologies enable comprehensive assessments of purity, potency, and immunogenicity.With biologics like monoclonal antibodies, fusion proteins, and recombinant hormones growing more complex, the need for precise testing of attributes such as glycosylation patterns, post-translational modifications, and aggregation is intensifying. Complementary techniques like high-performance liquid chromatography (HPLC) and size-exclusion chromatography (SEC) are widely employed to analyze stability and degradation pathways. The integration of automation and high-throughput screening platforms is also enhancing reproducibility and reducing testing variability, ultimately improving testing efficiency and quality.
Key Market Players
- Thermo Fisher Scientific Inc.
- Charles River Laboratories, Inc.
- SGS S.A.
- Eurofins Scientific Limited
- Intertek Group plc
- Element Materials Technology
- Pacific BioLabs, Inc.
- Sartorius AG
- WuXi AppTec
- Syngene International Ltd.
Report Scope:
In this report, the Global Biosimilar Testing Services Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Biosimilar Testing Services Market, By Service Type:
- Analytical Testing
- Clinical Testing
Biosimilar Testing Services Market, By Molecule Type:
- Monoclonal Antibodies
- Recombinant Hormones
- Insulin
- Interferons
- Enzymes
- Others
Biosimilar Testing Services Market, By Therapeutic Area:
- Oncology
- Autoimmune Diseases
- Diabetes
- Infectious Diseases
- Neurology
- Others
Biosimilar Testing Services Market, By End User:
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Academic & Research Institutes
- Others
Biosimilar Testing Services Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Biosimilar Testing Services Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Thermo Fisher Scientific Inc.
- Charles River Laboratories, Inc.
- SGS S.A.
- Eurofins Scientific Limited
- Intertek Group plc
- Element Materials Technology
- Pacific BioLabs, Inc.
- Sartorius AG
- WuXi AppTec
- Syngene International Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 181 |
| Published | July 2025 |
| Forecast Period | 2024 - 2030 |
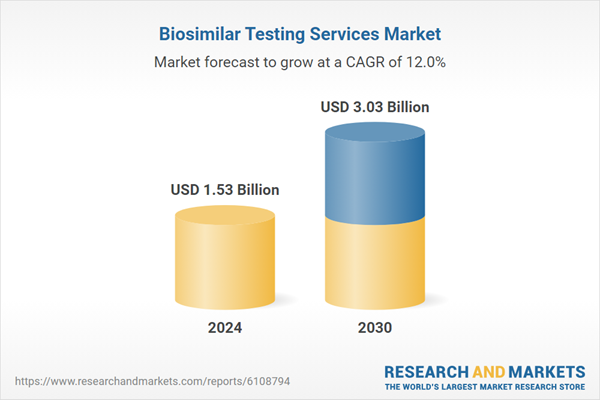
| Estimated Market Value ( USD | $ 1.53 Billion |
| Forecasted Market Value ( USD | $ 3.03 Billion |
| Compound Annual Growth Rate | 12.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |