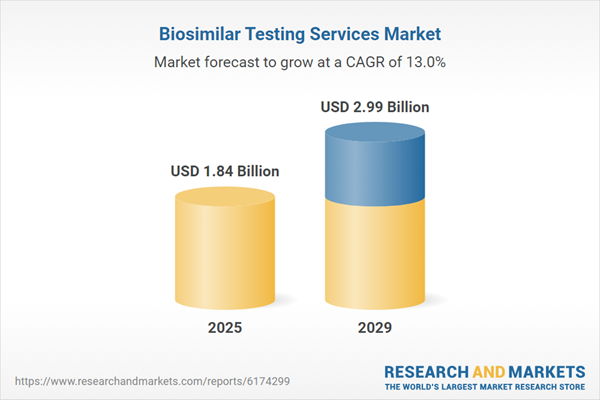
The biosimilar testing services market size is expected to see rapid growth in the next few years. It will grow to $2.99 billion in 2029 at a compound annual growth rate (CAGR) of 13%. The growth in the forecast period can be attributed to the increasing adoption of biosimilars, rising demand for cost-effective treatments, growing need for affordable biologic therapies, enhanced trust in biosimilar efficacy and safety, and greater demand for customized testing solutions. Major trends in the forecast period include advancements in analytical testing technologies, ongoing innovations in bioassay development, wider adoption of high-throughput testing platforms, increased research and development in biosimilar analytics, and the integration of artificial intelligence in data analysis.
The increasing prevalence of autoimmune diseases is expected to drive the growth of the biosimilar testing services market in the coming years. Autoimmune diseases occur when the body’s immune system mistakenly attacks its own healthy cells and tissues, causing inflammation and tissue damage. This rise is fueled by the growing need for effective biologic treatments that manage chronic symptoms, reduce flare-ups, and improve long-term patient outcomes. Biosimilar testing services support the treatment of autoimmune diseases by ensuring that biosimilars are safe, effective, and comparable to original biologics, providing affordable and accessible therapeutic options. For example, according to the Australian Institute of Health and Welfare, a government organization in Australia, an estimated 514,000 people (2%) were living with rheumatoid arthritis in 2022, with 2.5% of females and 1.6% of males affected. Consequently, the growing prevalence of autoimmune diseases is driving the expansion of the biosimilar testing services market.
Key players in the biosimilar testing services market are focusing on facility expansion to introduce new technologies and capabilities that enhance productivity and competitiveness. Facility expansion involves enlarging or upgrading existing infrastructure to accommodate growing operational needs or future growth. In October 2023, Tanvex BioPharma USA Inc., a US-based biotechnology company, launched Tanvex CDMO, a state-of-the-art contract development and manufacturing organization in San Diego. The facility offers end-to-end biologics development and manufacturing services, including cell line development, process optimization, analytical testing, and regulatory support. This expansion aims to accelerate the advancement of biopharmaceutical products from concept to commercial scale, leveraging advanced technologies and experienced teams to deliver innovative and life-changing biologics to patients worldwide.
In March 2022, Biocon Biologics Limited, an India-based biosimilars company, acquired the biosimilar portfolio of Viatris Inc. for $3.3 billion. Through this acquisition, Biocon Biologics seeks to establish a globally integrated biosimilars enterprise by combining Viatris’ biosimilar assets with its own pipeline and commercial capabilities, expanding access to affordable, high-quality biosimilar drugs worldwide. Viatris Inc. is a US-based healthcare company that provides biosimilars.
Major players in the biosimilar testing services market are Thermo Fisher Scientific Inc., IQVIA Laboratories, Laboratory Corporation of America Holdings, Fresenius SE & Co. KGaA, Eurofins Scientific SE, Intas Pharmaceuticals Ltd., SGS Société Générale de Surveillance SA, WuXi AppTec Co. Ltd., Intertek Group plc, Samsung Biologics Co. Ltd., Celltrion Inc., Almac Group Limited, Syngene International Limited, Shanghai Medicilon Inc., Kymos Group S.L., Pacific BioLabs Inc., Profacgen Inc., ExcellGene SA, BioPharmaSpec Ltd., and Sartorius Stedim Biotech Group.
North America was the largest region in the biosimilar testing services market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in biosimilar testing services market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the biosimilar testing services market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the consequent trade frictions in spring 2025 are severely impacting the healthcare sector, particularly in the supply of critical medical devices, diagnostic equipment, and pharmaceuticals. Hospitals and healthcare providers are facing higher costs for imported surgical instruments, imaging equipment, and consumables such as syringes and catheters, many of which have limited domestic alternatives. These increased costs are straining healthcare budgets, leading some providers to delay equipment upgrades or pass on expenses to patients. Additionally, tariffs on raw materials and components are disrupting the production of essential drugs and devices, causing supply chain bottlenecks. In response, the industry is diversifying sourcing strategies, boosting local manufacturing where possible, and advocating for tariff exemptions on life-saving medical products.
Biosimilar testing services refer to the scientific and analytical procedures performed to evaluate the similarity between a biosimilar and its reference biologic product. These services involve thorough assessment of structural, functional, and biological attributes to ensure consistency, quality, and safety. The primary objective is to confirm that the biosimilar demonstrates comparable efficacy and immunogenicity to the reference product while meeting regulatory requirements.
The primary types of biosimilar testing services include analytical testing, bioanalytical testing, stability testing, method development and validation, and others. Analytical testing encompasses a range of laboratory techniques used to evaluate the structural and functional similarity of biosimilars to reference biologics, ensuring product safety, quality, and effectiveness. These services utilize technologies such as cell-based assays, chromatography, mass spectrometry, and bioassays. The molecules tested include monoclonal antibodies, recombinant hormones, insulin, interferons, enzymes, and others. Applications span oncology, autoimmune diseases, blood disorders, growth hormone deficiency, and more, serving end users such as pharmaceutical and biotechnology companies, contract research organizations, academic research institutes, and government agencies.
The biosimilar testing services market research report is one of a series of new reports that provides biosimilar testing services market statistics, including the biosimilar testing services industry global market size, regional shares, competitors with the biosimilar testing services market share, detailed biosimilar testing services market segments, market trends, and opportunities, and any further data you may need to thrive in the biosimilar testing services industry. This biosimilar testing services market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The biosimilar testing services market consists of revenues earned by entities by providing services such as comparability testing, structural characterization, functional characterization, impurity profiling, glycosylation analysis, pharmacokinetic studies, immunogenicity assessment, and regulatory compliance support. The market value includes the value of related goods sold by the service provider or included within the service offering. The biosimilar testing services market also includes sales of reference biologics, biosimilar candidates, assay kits, analytical reagents, detection reagents, and bioanalytical instruments. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Biosimilar Testing Services Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on biosimilar testing services market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for biosimilar testing services? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The biosimilar testing services market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Service Type: Analytical Testing; Bioanalytical Testing; Stability Testing; Method Development and Validation; Other Service Types2) By Technology: Cell-Based Assays; Chromatography Techniques; Mass Spectrometry; Bioassays
3) By Molecule Type: Monoclonal Antibodies; Recombinant Hormones; Insulin; Interferons; Enzymes; Other Molecule Types
4) By Application: Oncology; Autoimmune Diseases; Blood Disorders; Growth Hormone Deficiency; Other Applications
5) By End User: Pharmaceutical Companies; Biotechnology Companies; Contract Research Organizations; Academic Research Institutes; Government Agencies
Subsegments:
1) By Analytical Testing: Protein Characterization; Impurity Profiling; Potency Testing; Glycosylation Analysis; Structural Analysis2) By Bioanalytical Testing: Pharmacokinetics Analysis; Immunogenicity Testing; Biomarker Analysis; Drug Concentration Measurement; Toxicokinetics Testing
3) By Stability Testing: Long-Term Stability Testing; Accelerated Stability Testing; Stress Testing; Photostability Testing; Temperature Cycling Studies
4) By Method Development and Validation: Analytical Method Development; Bioanalytical Method Development; Method Validation; Process Validation; Assay Development
5) By Other Service Types: Immunogenicity Assessment; Formulation Development; Microbial Testing; Release Testing; Regulatory Consulting
Companies Mentioned: Thermo Fisher Scientific Inc.; IQVIA Laboratories; Laboratory Corporation of America Holdings; Fresenius SE & Co. KGaA; Eurofins Scientific SE; Intas Pharmaceuticals Ltd.; SGS Société Générale de Surveillance SA; WuXi AppTec Co. Ltd.; Intertek Group plc; Samsung Biologics Co. Ltd.; Celltrion Inc.; Almac Group Limited; Syngene International Limited; Shanghai Medicilon Inc.; Kymos Group S.L.; Pacific BioLabs Inc.; Profacgen Inc.; ExcellGene SA; BioPharmaSpec Ltd.; Sartorius Stedim Biotech Group.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Biosimilar Testing Services market report include:- Thermo Fisher Scientific Inc.
- IQVIA Laboratories
- Laboratory Corporation of America Holdings
- Fresenius SE & Co. KGaA
- Eurofins Scientific SE
- Intas Pharmaceuticals Ltd.
- SGS Société Générale de Surveillance SA
- WuXi AppTec Co. Ltd.
- Intertek Group plc
- Samsung Biologics Co. Ltd.
- Celltrion Inc.
- Almac Group Limited
- Syngene International Limited
- Shanghai Medicilon Inc.
- Kymos Group S.L.
- Pacific BioLabs Inc.
- Profacgen Inc.
- ExcellGene SA
- BioPharmaSpec Ltd.
- Sartorius Stedim Biotech Group.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.84 Billion |
| Forecasted Market Value ( USD | $ 2.99 Billion |
| Compound Annual Growth Rate | 13.0% |
| Regions Covered | Global |
| No. of Companies Mentioned | 21 |