Global Microchips in Medicine Market - Key Trends & Drivers Summarized
How Are Microchips Transforming the Future of Medical Diagnostics and Therapeutics?
Microchips in medicine-also known as implantable or ingestible medical chips-are redefining how healthcare professionals diagnose, monitor, and treat a wide array of health conditions. These semiconductor-based devices, often the size of a grain of rice or smaller, are being used to monitor vital signs, deliver drugs at targeted locations, or verify patient adherence to prescribed regimens. Ingestible sensor chips embedded in pills can transmit data once ingested, providing real-time feedback on medication intake. Meanwhile, implantable chips monitor chronic diseases such as cardiovascular disorders, epilepsy, or diabetes through long-term biosignal tracking.These chips represent a fusion of biocompatible materials, micro-electromechanical systems (MEMS), low-power semiconductors, and wireless data transmission. Unlike traditional monitoring techniques that require hospital visits or bulky equipment, microchips offer continuous, passive, and remote health surveillance. As global healthcare pivots toward personalized medicine, these chips enable physicians to adjust treatment regimens based on patient-specific data collected in real time-minimizing adverse drug reactions and maximizing therapeutic efficacy.
What Technological Breakthroughs Are Driving Adoption of Microchips in Healthcare?
Significant innovations in materials science, nanofabrication, and microfluidics are propelling the deployment of microchips in medical settings. Chips now feature biosensors capable of detecting biochemical signals such as pH, glucose levels, electrolytes, or enzyme activity. Power efficiency has been vastly improved, allowing chips to function for months or even years using miniature batteries or energy harvesting mechanisms (e.g., piezoelectric or thermoelectric systems). Ultra-low-power integrated circuits (ICs) and wireless systems-on-chip (SoCs) enable seamless data transmission to smartphones or cloud platforms.One of the most promising areas is smart drug delivery systems, wherein chips are pre-programmed to release drugs in response to biological triggers or via remote control. This has been validated in preclinical studies for cancer treatment and hormone therapy. Another area of rapid growth is biosignal telemetry, where microchips embedded in pacemakers or neurostimulators relay continuous data on heart rhythms or neural activity. Recent developments also include skin-interfaced microchips and electronic tattoos capable of non-invasively tracking hydration, temperature, and electrophysiological signals.
Furthermore, blockchain-based secure data transmission and AI-driven analytics are making chip-generated data actionable in predictive diagnostics and population health management. Regulatory bodies such as the FDA are increasingly acknowledging the safety and utility of such chips, thereby accelerating clinical adoption.
Which Medical Fields Are Seeing the Highest Adoption Rates of Microchip Technologies?
Chronic disease management is among the most significant application areas. Diabetic patients, for instance, benefit from continuous glucose monitors (CGMs) equipped with microchips, enabling non-stop data relay to insulin pumps or healthcare providers. In cardiology, implantable chips inside pacemakers or defibrillators are used to track arrhythmias, heart rate variability, and early signs of heart failure. Neurological applications include chips that monitor seizures, intracranial pressure, or spinal cord activity in real time.The pharmaceutical sector is integrating ingestible microchips into drug trials and compliance monitoring. Proteus Digital Health pioneered this segment, launching a pill that signals successful ingestion-a feature being utilized to verify adherence in schizophrenia and hepatitis C treatments. The oncology field is testing microchips for intra-tumoral drug delivery or thermal ablation guidance. In orthopedic and prosthetic applications, chips are being embedded in implants to monitor healing rates, load dynamics, and infection markers.
Veterinary medicine is another parallel growth segment where RFID microchips are used for tracking, diagnostics, and vaccine monitoring in livestock and pets. Geographically, North America leads in R&D, clinical trials, and commercialization, followed by Europe with a strong focus on patient data regulation and public health integration. Asia-Pacific-particularly China, Japan, and South Korea-is emerging rapidly due to government initiatives in digital health infrastructure and semiconductor innovation.
What Is Fueling Growth in the Global Microchips in Medicine Market?
The growth in the global microchips in medicine market is driven by several factors, including the rising burden of chronic diseases, a shift toward patient-centric healthcare delivery, and increased adoption of remote monitoring technologies. The demand for real-time, continuous diagnostics has prompted healthcare systems to integrate microchips into mainstream treatment pathways. Miniaturization and affordability of sensors and chips are reducing entry barriers, enabling broader deployment across both inpatient and outpatient settings.A parallel rise in the use of smartphones, wearables, and IoT platforms in healthcare is creating a supportive digital ecosystem for chip-enabled diagnostics. The COVID-19 pandemic accelerated telehealth acceptance and highlighted the need for contactless monitoring-reinforcing the value of ingestible or implantable microchips. Pharmaceutical companies are increasingly embedding chips in oral formulations to improve drug compliance, especially in mental health and infectious disease segments.
The healthcare sector’s movement toward outcome-based reimbursement and preventative care further incentivizes continuous monitoring tools that microchips offer. Reimbursement codes for digital therapeutics and remote diagnostics are expanding across the US, UK, and parts of Europe, bolstering commercial viability. As concerns over data privacy and biocompatibility are addressed through secure architectures and inert materials, the microchip-enabled healthcare future continues to gain institutional, technological, and market momentum.
Scope of the Report
The report analyzes the Microchips in Medicine market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Application (Drug Delivery Application, Home Monitoring Application, Other Applications); End-Use (Hospitals End-Use, Research Centers End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Drug Delivery Application segment, which is expected to reach US$1.8 Billion by 2030 with a CAGR of a 12.2%. The Home Monitoring Application segment is also set to grow at 8.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $401.3 Million in 2024, and China, forecasted to grow at an impressive 14.9% CAGR to reach $569.1 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Microchips in Medicine Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Microchips in Medicine Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Microchips in Medicine Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Becton, Dickinson and Company (BD), BioTelemetry (a Philips company), Biotronik, Boston Scientific Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this Microchips in Medicine market report include:
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- BioTelemetry (a Philips company)
- Biotronik
- Boston Scientific Corporation
- Capsule Technologies (A Qualcomm Life Company)
- Cepheid (a Danaher company)
- Cochlear Limited
- Dexcom Inc.
- GE HealthCare
- Hillrom (now part of Baxter)
- Honeywell Life Sciences
- Implantica
- Masimo Corporation
- Medtronic Plc
- Microchip Biotechnologies Inc.
- NXP Semiconductors
- PerkinElmer (now part of Revvity)
- Siemens Healthineers
- Zimmer Biomet
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Becton, Dickinson and Company (BD)
- BioTelemetry (a Philips company)
- Biotronik
- Boston Scientific Corporation
- Capsule Technologies (A Qualcomm Life Company)
- Cepheid (a Danaher company)
- Cochlear Limited
- Dexcom Inc.
- GE HealthCare
- Hillrom (now part of Baxter)
- Honeywell Life Sciences
- Implantica
- Masimo Corporation
- Medtronic Plc
- Microchip Biotechnologies Inc.
- NXP Semiconductors
- PerkinElmer (now part of Revvity)
- Siemens Healthineers
- Zimmer Biomet
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 285 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
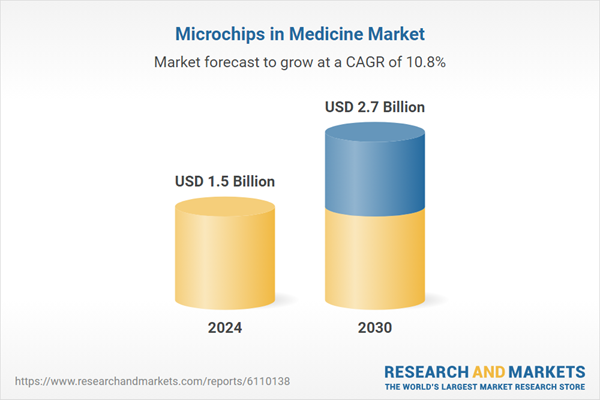
| Estimated Market Value ( USD | $ 1.5 Billion |
| Forecasted Market Value ( USD | $ 2.7 Billion |
| Compound Annual Growth Rate | 10.8% |
| Regions Covered | Global |