Global Esophageal Catheters Market - Key Trends & Drivers Summarized
How Are Esophageal Catheters Supporting Critical Respiratory and Diagnostic Monitoring?
Esophageal catheters are specialized medical devices inserted into the esophagus to monitor physiological parameters such as pressure, temperature, and pH. These catheters are essential in intensive care and surgical settings, where they provide indirect but accurate measurements of respiratory mechanics, gastric acid exposure, or core body temperature. They are particularly useful in mechanically ventilated patients, where esophageal pressure monitoring helps guide ventilator settings and assess lung compliance.Beyond respiratory care, esophageal pH and impedance catheters are used in gastroenterology to diagnose gastroesophageal reflux disease (GERD), esophageal motility disorders, and non-cardiac chest pain. These catheters capture real-time data on reflux events and acid exposure duration, supporting precise diagnosis and tailored treatment strategies. Their use is expanding due to increasing clinical awareness of esophageal function and its link to respiratory and digestive disorders.
What Technological Advancements Are Improving Accuracy and Patient Tolerance?
Modern esophageal catheters are designed to be minimally invasive and patient-friendly. They feature soft, biocompatible materials and flexible designs that reduce discomfort during placement and prolonged monitoring. Multi-sensor catheters with multiple pressure or impedance sensors provide high-resolution data across the esophagus, enhancing diagnostic accuracy and waveform interpretation.Wireless pH monitoring systems, such as capsule-based alternatives, are also gaining adoption for their ability to perform extended monitoring without transnasal tubing. In respiratory monitoring, integration with advanced ventilator systems and data platforms enables real-time visualization of pressure-volume relationships, improving ventilatory strategy personalization. These technological refinements are increasing clinician confidence in using esophageal catheters as standard diagnostic and monitoring tools.
Which Clinical Applications and User Settings Are Expanding Utilization?
Esophageal catheters are used in critical care units, surgical theaters, and gastrointestinal diagnostic labs. In intensive care, they assist anesthesiologists and pulmonologists in monitoring transpulmonary pressure to optimize mechanical ventilation and prevent lung injury. During thoracic and cardiac surgeries, catheters enable precise temperature monitoring and hemodynamic assessment.In outpatient and ambulatory care, gastroenterologists use esophageal impedance and pH catheters to perform 24-hour reflux studies and motility assessments. Pediatric applications are also growing, particularly in diagnosing reflux or swallowing disorders. The availability of disposable, sterile, single-use catheters is improving hygiene standards and enabling broader usage across institutions with varied infection control protocols.
What Is Driving Growth in the Esophageal Catheters Market?
Growth in the esophageal catheters market is driven by several factors related to respiratory care, gastrointestinal diagnostics, and intensive care monitoring. Increased use of mechanical ventilation in critical care, especially during respiratory pandemics and complex surgeries, is expanding demand for esophageal pressure monitoring. Rising diagnosis rates of GERD and motility disorders are supporting the use of pH and impedance catheters in clinical gastroenterology.Advancements in catheter design and multi-sensor configurations are enhancing diagnostic precision and patient tolerance. Greater awareness among clinicians of the diagnostic value of esophageal metrics is prompting inclusion in care protocols, particularly in respiratory therapy and gastroenterology. As minimally invasive, data-rich diagnostics become more central to personalized medicine, esophageal catheters are gaining traction as tools that bridge critical care, surgical monitoring, and gastrointestinal assessment.
Scope of the Report
The report analyzes the Esophageal Catheters market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Type (Balloon Dilation Catheter, Irrigation Catheter, Pressure Monitoring Catheter); Application (Gastroesophageal Reflux Disease Application, Dysphagia Application, Chest Pain Application, Other Applications); End-User (Hospitals End-User, Clinics End-User, Ambulatory Surgery Centers End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Balloon Dilation Catheter segment, which is expected to reach US$2.1 Billion by 2030 with a CAGR of a 6.3%. The Irrigation Catheter segment is also set to grow at 7.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $719.1 Million in 2024, and China, forecasted to grow at an impressive 10.5% CAGR to reach $808.6 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Esophageal Catheters Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Esophageal Catheters Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Esophageal Catheters Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Avanos Medical Inc., B. Braun Melsungen AG, Becton, Dickinson and Company (BD), Boston Scientific Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Esophageal Catheters market report include:
- Abbott Laboratories
- Avanos Medical Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cook Medical Inc.
- Edwards Lifesciences Corporation
- FUJIFILM Medical Systems
- Hamilton Medical AG
- Japan Lifeline Co. Ltd.
- Laborie Medical Technologies Inc.
- Medela AG
- Medline Industries LP
- Medtronic plc
- Merit Medical Systems Inc.
- Olympus Corporation
- Pentax Medical
- Stryker Corporation
- Teleflex Incorporated
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Avanos Medical Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Cook Medical Inc.
- Edwards Lifesciences Corporation
- FUJIFILM Medical Systems
- Hamilton Medical AG
- Japan Lifeline Co. Ltd.
- Laborie Medical Technologies Inc.
- Medela AG
- Medline Industries LP
- Medtronic plc
- Merit Medical Systems Inc.
- Olympus Corporation
- Pentax Medical
- Stryker Corporation
- Teleflex Incorporated
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 372 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
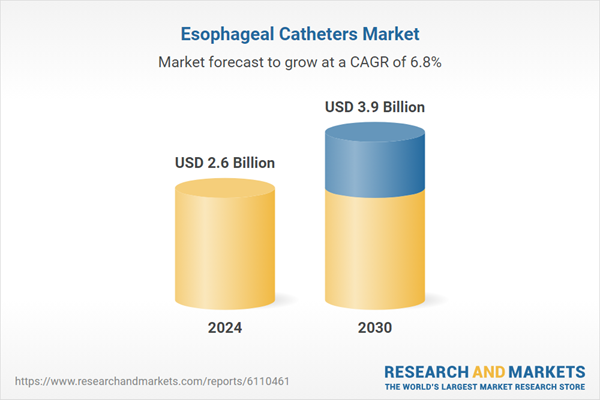
| Estimated Market Value ( USD | $ 2.6 Billion |
| Forecasted Market Value ( USD | $ 3.9 Billion |
| Compound Annual Growth Rate | 6.8% |
| Regions Covered | Global |