Global Activity-Dependent Neuroprotective Protein (ADNP) Syndrome Treatment Market - Key Trends & Drivers Summarized
What Makes ADNP Syndrome a Unique Challenge in the Landscape of Rare Neurological Disorders?
Activity-Dependent Neuroprotective Protein (ADNP) syndrome represents one of the more complex and recently recognized rare neurodevelopmental disorders, characterized by mutations in the ADNP gene that significantly disrupt brain development and function. Children diagnosed with this syndrome often exhibit a broad spectrum of symptoms including intellectual disability, autism spectrum disorder (ASD), delayed speech and motor skills, hypotonia, sleep disturbances, and sometimes seizures. As ADNP plays a critical role in brain formation, synaptic function, and cognitive processes, its deficiency or mutation leads to widespread neurological dysfunction from early developmental stages. The rarity of the condition, combined with its heterogeneous presentation, poses substantial challenges in diagnosis and treatment. Physicians often struggle to differentiate ADNP syndrome from other autism-related disorders, and many cases remain underdiagnosed or misdiagnosed. The lack of disease-specific therapies also limits clinicians to symptomatic management strategies such as behavioral therapies, speech and occupational therapy, and pharmacological interventions for co-existing conditions like anxiety or epilepsy. This has created an urgent unmet need within the medical community for targeted therapies that can address the root cause of the syndrome. The growing recognition of ADNP syndrome by geneticists, neurologists, and pediatric specialists has, however, led to increased efforts in genetic screening and awareness campaigns. Patient advocacy groups are also playing a significant role in accelerating research, securing funding, and fostering international collaboration to better understand the pathology and therapeutic potential of this rare disorder. As awareness and diagnosis improve, the urgency for developing effective treatment pathways becomes more pressing in the rare disease ecosystem.Why Is Research Into Targeted ADNP Syndrome Treatment Gaining Momentum Across the Biomedical Sector?
Research into targeted treatments for ADNP syndrome is gaining considerable traction across the biomedical sector due to advances in genomics, the identification of molecular pathways affected by ADNP mutations, and increasing interest in rare diseases by pharmaceutical innovators. One of the central drivers of this momentum is the growing ability of researchers to analyze the specific gene disruptions involved in ADNP syndrome and correlate them with clinical manifestations. This genotype-phenotype mapping is enabling the development of more personalized therapeutic approaches. Additionally, the discovery that ADNP plays a role in the regulation of over 400 other genes has highlighted its broader importance in neurodevelopment and made it a promising target for pharmacological intervention. Peptide-based therapies, such as the synthetic peptide NAP (davunetide), which mimics ADNP’s neuroprotective functions, have emerged as a potential treatment pathway. Preclinical studies have demonstrated NAP’s ability to enhance cognitive function, stabilize microtubules, and improve neuronal communication, making it a focal point in current drug development pipelines. Biotechnology startups and academic research centers are increasingly collaborating to fast-track preclinical validation and clinical trials, often supported by patient registries and real-world data collected through caregiver networks. Regulatory agencies are also encouraging innovation in this area through orphan drug designations, which provide financial and logistical incentives for developers targeting rare conditions. Moreover, the rise of gene therapy and antisense oligonucleotide platforms has opened new doors for correcting or compensating for faulty gene expression in ADNP-related conditions. As scientific tools and genetic insights advance, the research landscape around ADNP syndrome is shifting from passive observation to proactive therapeutic discovery.What Treatment Strategies Are Currently Used and How Are They Evolving to Address ADNP Syndrome?
Current treatment strategies for ADNP syndrome are largely symptomatic and supportive, focusing on improving quality of life and developmental outcomes through multidisciplinary care approaches. These include early intervention programs incorporating speech therapy, occupational therapy, and physical therapy to address delays in communication, motor skills, and coordination. Behavioral therapies, particularly those used in autism spectrum disorder management such as Applied Behavior Analysis (ABA), are often employed to address social and behavioral challenges associated with the syndrome. Pharmacologic interventions are used to manage secondary conditions like sleep disturbances, seizures, gastrointestinal issues, and anxiety, although these do not target the root cause of the disorder. Because of the variability in symptom severity, treatment plans must be highly individualized, requiring regular assessments and adjustments. In parallel with traditional care models, families are increasingly turning to integrative approaches including nutritional interventions, sensory therapies, and alternative communication methods to support developmental progress. As research progresses, new strategies are being explored that aim to directly address the molecular dysfunction caused by ADNP mutations. This includes the use of neuroprotective peptides such as NAP, which has shown promise in improving behavioral and cognitive symptoms in preclinical studies. Advances in gene editing technologies like CRISPR, while still in early stages, present a theoretical path for future curative interventions. Ongoing clinical trials and patient registries are helping to build an evidence base for potential therapies, allowing for a more nuanced understanding of disease progression and therapeutic efficacy. Overall, the evolution of treatment is moving toward more personalized, molecularly informed models that aim not just to manage but to modify the course of ADNP syndrome.What Are the Key Factors Driving Global Efforts Toward ADNP Syndrome Treatment Development?
The global push toward developing effective treatments for ADNP syndrome is driven by several interconnected factors, beginning with the increasing awareness and diagnostic recognition of the condition. As more pediatric and genetic clinics adopt advanced sequencing techniques, particularly whole exome and genome sequencing, more cases of ADNP syndrome are being accurately diagnosed, creating a clearer picture of the patient population and the disease burden. The emergence of patient advocacy groups, such as the ADNP Kids Research Foundation, has played a pivotal role in funding early-stage research, supporting families, and pushing for policy changes that prioritize rare disease innovation. The designation of ADNP syndrome as a rare or orphan disease in many jurisdictions provides incentives to pharmaceutical developers, including tax credits, market exclusivity, and fast-track regulatory pathways, which are accelerating therapeutic research and development. The scientific validation of ADNP’s role in regulating numerous genes associated with brain development and neuroprotection has attracted academic interest and biotech investments, positioning the condition within broader neurodevelopmental research frameworks. Advances in precision medicine, including gene therapies, peptide therapeutics, and RNA-targeted drugs, are offering new tools to develop treatments that could potentially alter the disease’s progression. The availability of patient registries, natural history studies, and caregiver-reported outcomes is also making it easier to design and implement clinical trials with measurable endpoints. Additionally, the increasing focus on pediatric neurological disorders by global health organizations is fostering greater collaboration and funding. Together, these elements are creating an ecosystem that supports rapid discovery, translational research, and eventual clinical adoption of new therapies aimed at transforming the treatment landscape for ADNP syndrome.Scope of the Report
The report analyzes the Activity-Dependent Neuroprotective Protein (ADNP) Syndrome Treatment market, presented in terms of market value (USD). The analysis covers the key segments and geographic regions outlined below:- Segments: Treatment Type (Therapies, Medications, Surgeries, Other Treatment Types); End-User (Research Institutes End-User, Research Laboratories End-User, Hospitals End-User).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Therapies segment, which is expected to reach US$21.8 Million by 2030 with a CAGR of a 8.5%. The Medications segment is also set to grow at 4.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $9.2 Million in 2024, and China, forecasted to grow at an impressive 11.2% CAGR to reach $10.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Activity-Dependent Neuroprotective Protein (ADNP) Syndrome Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Activity-Dependent Neuroprotective Protein (ADNP) Syndrome Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Activity-Dependent Neuroprotective Protein (ADNP) Syndrome Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as ADNP Kids Research Foundation, Alexion Pharmaceuticals, Alnylam Pharmaceuticals, Butterfly Effects, Coronis Neurosciences and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 44 companies featured in this Activity-Dependent Neuroprotective Protein (ADNP) Syndrome Treatment market report include:
- ADNP Kids Research Foundation
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals
- Butterfly Effects
- Coronis Neurosciences
- Curemark
- Easterseals
- Hopebridge, LLC
- Icahn School of Medicine at Mount Sinai
- LEARN Behavioral
- May Institute
- Neuropathix
- Neurocrine Biosciences
- Prevail Therapeutics (Eli Lilly)
- Simba Global
- TauRx Therapeutics
- Tetraneuron
- The University of Alabama at Birmingham
- UC Regents / UC Davis MIND Institute
- The Watson Institute
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025 (E), competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- ADNP Kids Research Foundation
- Alexion Pharmaceuticals
- Alnylam Pharmaceuticals
- Butterfly Effects
- Coronis Neurosciences
- Curemark
- Easterseals
- Hopebridge, LLC
- Icahn School of Medicine at Mount Sinai
- LEARN Behavioral
- May Institute
- Neuropathix
- Neurocrine Biosciences
- Prevail Therapeutics (Eli Lilly)
- Simba Global
- TauRx Therapeutics
- Tetraneuron
- The University of Alabama at Birmingham
- UC Regents / UC Davis MIND Institute
- The Watson Institute
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 284 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
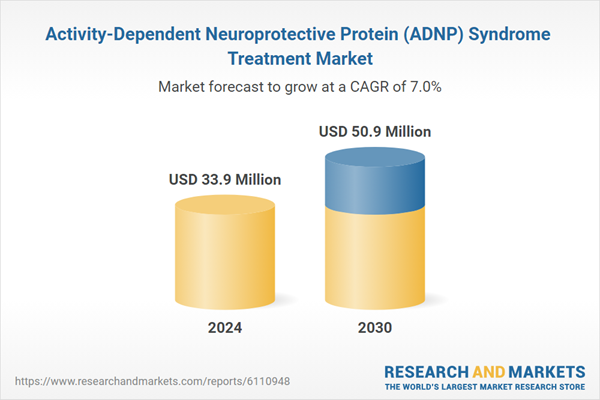
| Estimated Market Value ( USD | $ 33.9 Million |
| Forecasted Market Value ( USD | $ 50.9 Million |
| Compound Annual Growth Rate | 7.0% |
| Regions Covered | Global |