U.S. STI & Vaginitis PCR Testing Market Trends
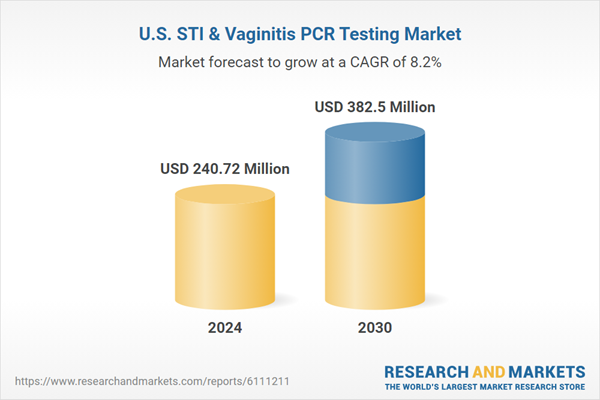
The U.S. STI & vaginitis PCR testing market size was estimated at USD 240.72 million in 2024 and is projected to grow at a CAGR of 8.2% from 2025 to 2030. The increasing prevalence of sexually transmitted infections and growing patient awareness are driving demand for advanced diagnostic tools. The Centers for Disease Control and Prevention (CDC) continues to report millions of new STI cases each year, with chlamydia, gonorrhea, and trichomoniasis among the most reported. This rise in infections has created a pressing need for early and accurate detection methods. Polymerase chain reaction (PCR) tests provide high sensitivity and specificity, offering a reliable alternative to traditional diagnostics. As patients and clinicians prioritize quick and accurate results, PCR testing is gaining widespread preference in urban populations, with better access to healthcare facilities and higher testing frequencies, further supporting market expansion. The gradual decline in stigma related to STI screening also plays a vital role in encouraging timely diagnosis.The integration of PCR testing into routine gynecological and sexual health evaluations in clinics and hospitals is another key driver. Many healthcare providers now recommend regular testing for at-risk populations, enhancing demand for PCR-based solutions. The growing trend of point-of-care testing and at-home sample collection kits has made STI and vaginitis testing more convenient. These innovations support early detection and reduce barriers associated with clinic-based testing. As a result, diagnostic labs and biotech firms are investing in developing user-friendly, rapid PCR kits tailored for the U.S. market. Rising partnerships between diagnostic companies and telehealth platforms expand access to PCR testing services. Increased healthcare spending by private insurers and employers also supports wider adoption of advanced diagnostic technologies.
Technological advancements in multiplex PCR platforms are improving the efficiency of STI and vaginitis testing. These innovations allow the simultaneous detection of multiple pathogens from a single sample, reducing costs and turnaround time. Such developments are particularly valuable for managing co-infections, which are common among patients with STIs. Furthermore, ongoing research into microfluidic PCR devices and automated sample preparation enhances laboratory throughput. This progress benefits large diagnostic networks and independent U.S. labs facing rising testing volumes. The competitive landscape is also evolving, with leading players launching novel PCR assays targeting hard-to-detect organisms. As these advanced diagnostics become more accessible, the overall market will witness consistent revenue growth through 2030.
Trichomoniasis remains a significant concern, especially in women, with recent studies emphasizing its link to adverse pregnancy outcomes, prompting wider use of molecular testing. Herpes Simplex Virus (HSV-1 & HSV-2) continues to see widespread testing due to its recurrent nature and asymptomatic shedding, with new rapid PCR assays gaining FDA approval for quicker diagnosis. Human Papillomavirus (HPV) testing is essential for identifying high-risk strains linked to cervical and other cancers, as seen in the adoption of the latest FDA-approved HPV assays like Roche’s Cobas HPV test for early cancer screening. Syphilis and other STIs, though less common, are included in multiplex panels to ensure comprehensive detection and early intervention, with multiplex PCR panels recently validated in clinical studies for improved sensitivity.
U.S. STI & Vaginitis PCR Testing Market Report Segmentation
This report forecasts country revenue growth and analyzes the latest industry trends in each sub-segment from 2018 to 2030. For this study, the analyst has segmented the U.S. STI & vaginitis PCR testing market report based on condition, test type, and end use.Condition Outlook (Revenue, USD Million, 2018 - 2030)
- Sexually Transmitted Infections (STIs)
- Chlamydia
- Gonorrhea
- Trichomoniasis
- Herpes Simplex Virus (HSV-1 & HSV-2)
- Human Papillomavirus (HPV)
- Syphilis
- Other
- Vaginal Infections
- Bacterial Vaginosis
- Vulvovaginal Candidiasis
- Others
Test Type Outlook (Revenue, USD Million, 2018 - 2030)
- STI PCR Panels
- Vaginitis PCR Panels
End Use Outlook (Revenue, USD Million, 2018 - 2030)
- Hospitals and Clinics
- Diagnostic Laboratories
- Homecare/At-home Testing
- Others
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Becton, Dickinson and Com (BD)
- F. Hoffmann-La Roche Ltd.
- Hologic, Inc.
- Abbott
- Danaher Corporation (Cepheid)
- Seegene Inc.
- bioMérieux (BioFire Diagnostics)
- QIAGEN
- Thermo Fisher Scientific, Inc.
- DiaSorin S.p.A (Luminex)
- R-Biopharm AG
- altona Diagnostics GmbH
- CERTEST BIOTEC.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 100 |
| Published | June 2025 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 240.72 Million |
| Forecasted Market Value ( USD | $ 382.5 Million |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | United States |
| No. of Companies Mentioned | 13 |