Speak directly to the analyst to clarify any post sales queries you may have.
A concise and compelling introduction that frames hereditary angioedema clinical complexity, evolving therapeutic modalities, and strategic priorities for stakeholders
Hereditary angioedema remains a clinically complex and commercially dynamic rare disease area, characterized by episodic swelling events driven by dysregulated kallikrein-kinin pathways. Patients and clinicians now navigate a therapeutic landscape where symptomatic control, prevention of attacks, and quality-of-life improvements are all viable endpoints that influence prescribing and reimbursement decisions. As a result, manufacturers, payers, clinicians, and patient advocacy groups must align around evidence that demonstrates not only clinical efficacy but also practical considerations such as route of administration, treatment burden, and real-world safety profiles.Transitioning from historical, inpatient-centered models of care toward outpatient and home-based administration has re-shaped patient expectations and payer requirements. Simultaneously, the acceleration of novel modalities-ranging from targeted small molecules to monoclonal antibodies and next-generation biologics-has intensified competition while broadening therapeutic options. This introductory analysis synthesizes clinical trends, commercial dynamics, and policy drivers to equip decision-makers with a structured view of the challenges and opportunities that define contemporary HAE strategy. It foregrounds the interplay between clinical differentiation and access pathways and sets the context for the deeper section-level insights that follow.
How advances in drug design, delivery innovations, and regulatory emphasis on real-world evidence are reshaping clinical care and commercial strategies in HAE therapeutics
The past several years have seen transformative shifts that are reshaping how hereditary angioedema is treated, managed, and reimbursed. Innovations in drug design and delivery have driven a movement away from exclusively intravenous therapies toward subcutaneous and oral options that prioritize convenience, adherence, and outpatient care. This shift has downstream effects on where care is delivered, how payers evaluate value, and how manufacturers design patient support programs. Moreover, advances in molecular understanding of the kallikrein-kinin cascade have enabled more selective targeting, creating therapeutic niches for agents differentiated by mechanism of action, onset of effect, and durability.Concurrently, stakeholders are responding to evolving regulatory expectations that emphasize real-world evidence and long-term safety monitoring, prompting clinical development programs to incorporate pragmatic endpoints and patient-reported outcomes. Commercially, the field has moved from single-product dominance in many geographies to a more competitive environment where pipeline entrants, biosimilar entrants, and alternative modalities compete on convenience, safety, and total cost of care. These converging trends require companies to adopt integrated development-to-commercialization strategies that anticipate payer scrutiny, emphasize differentiated clinical value, and deploy patient-centric delivery models to maintain access and uptake.
Assessing the operational and commercial repercussions of recent United States tariff changes on supply chains, payer dynamics, and patient access for HAE therapies
Recent trade policy developments, including tariff adjustments affecting pharmaceutical inputs and finished products, create a layered set of operational and commercial implications for HAE therapies distributed in and through the United States. Import-dependent supply chains for raw materials, biologic components, and certain finished formulations can experience cost pressure and logistical friction, prompting manufacturers to reassess sourcing strategies and inventory buffers. The cumulative effect of tariffs interacts with existing complexities in biologics production, cold-chain requirements, and regulatory lot-release processes to heighten the importance of supply-chain resilience.From a commercial perspective, increased input costs can compress margins or necessitate downstream contractual negotiations with payers and distributors to sustain manufacturer economics. Payer bodies and integrated delivery networks may respond with heightened formulary scrutiny, utilization management, and preference for therapies that demonstrate lower total cost of care through reduced hospitalizations or emergency interventions. In turn, manufacturers should prioritize scenario planning that models tariff-induced cost volatility, accelerate local or nearshore manufacturing where feasible, and enhance transparency with payers about cost drivers. Policy engagement and industry-level advocacy may mitigate some tariff impacts, while targeted patient access programs can preserve treatment continuity for vulnerable populations during periods of supply or cost disruption.
Segment-focused insights across indication types, drug classes, administration routes, distribution channels, and patient age groups that determine clinical and commercial differentiation
A nuanced segmentation lens reveals differentiated drivers across indication, drug class, route of administration, distribution channel, and patient age that collectively shape clinical uptake and commercial performance. When considering indication type, acute treatment strategies are evaluated on rapidity of onset and ease of administration, with categories spanning bradykinin receptor antagonists, C1 inhibitors, and kallikrein inhibitors; within these, C1 inhibitors split into plasma-derived and recombinant formulations while kallikrein inhibitors bifurcate into monoclonal antibodies and small molecule agents. Long-term prophylaxis has its own value calculus, where sustained efficacy, convenience of dosing, and safety for chronic use determine adoption, and the same subdivisions of C1 inhibitor and kallikrein inhibitor modalities apply with plasma-derived and recombinant, and monoclonal and small molecule distinctions respectively.Drug class segmentation underscores how mechanism-specific attributes inform positioning; bradykinin receptor antagonists are often judged by speed and short-term symptom control, whereas kallikrein-targeted agents and C1 inhibitors are assessed for prophylactic durability and safety. Route of administration remains a primary differentiator: intravenous formulations carry procedural and infusion-center considerations, subcutaneous options enable self-administration and home-based care, and oral therapies prioritize convenience and adherence but must demonstrate robust efficacy and tolerability. Distribution channels further mediate access, as hospital pharmacies, retail pharmacies, and specialty pharmacies each present distinct reimbursement, stocking, and patient-support models that influence prescribing behavior. Finally, patient age group considerations separate adult and pediatric pathways, requiring tailored dosing regimens, safety monitoring, and caregiver support frameworks that affect product positioning and clinical trial design.
How regional regulatory diversity, payer ecosystems, and distribution infrastructure across Americas, Europe Middle East & Africa, and Asia-Pacific shape access and commercialization strategies
Regional dynamics materially affect regulatory timelines, patient access, and commercialization tactics across the Americas, Europe, Middle East & Africa, and Asia-Pacific. In the Americas, established reimbursement frameworks and a strong culture of specialty pharmacy integration enable rapid adoption of differentiated therapies, but payer negotiations and prior authorization requirements can create access barriers that demand proactive evidence generation and pricing strategies. Moving to Europe, Middle East & Africa, heterogeneous regulatory environments and national reimbursement decisions require tailored market-entry plans that reflect localized HTA expectations, pricing constraints, and distribution partner capabilities. Manufacturers must align dossiers with region-specific evidentiary standards and pair clinical messaging with cost-effectiveness narratives.Asia-Pacific presents a mosaic of opportunities and constraints, where high unmet need and growing specialty care infrastructure coexist with variable regulatory pathways and pricing pressures. In many jurisdictions within this region, early engagement with local stakeholders, investments in local study data, and collaborations with healthcare systems accelerate market access. Across all regions, clinical trial site selection, real-world evidence collection, and patient support offerings should be adapted to local care delivery models. Strategic regional prioritization balances regulatory complexity, payer receptivity, manufacturing logistics, and the availability of specialty distribution networks that influence the speed and scale of uptake.
Strategic company positioning and partnership dynamics that determine competitive advantage, manufacturing resilience, and commercialization success in HAE therapeutics
Competitive landscapes in HAE are populated by a mix of established biologics manufacturers, specialty pharmaceutical companies, and agile biotech innovators that prioritize either differentiated mechanism-of-action profiles or novel delivery formats. Incumbent companies often leverage manufacturing scale, broad specialty distribution relationships, and deep patient support programs to defend position, while emergent firms compete on clinical differentiation, patent life, and the potential for improved convenience through subcutaneous or oral formulations. Partnerships and strategic alliances play a central role in accelerating development timelines, accessing new markets, and augmenting manufacturing capacity, particularly for complex biologic modalities.Intellectual property strategies, pipeline breadth, and demonstrated post-market safety are key axes by which companies differentiate themselves with payers and clinicians. Contract manufacturing organizations and specialist biologics manufacturers are increasingly important partners due to their role in ensuring supply continuity and enabling localized production. From a commercial perspective, companies that integrate robust real-world evidence programs, invest in digital patient support, and align pricing strategies with demonstrable reductions in acute-care utilization will be better positioned to navigate formulary processes and achieve sustainable uptake. Strategic M&A and licensing activity will likely continue as firms seek to fill portfolio gaps and accelerate access to emerging modalities.
Actionable strategic recommendations for manufacturers, payers, and providers to secure access, manage cost pressures, and accelerate adoption of differentiated HAE therapies
Industry leaders should prioritize a set of concerted actions that align clinical innovation with durable access pathways and scalable commercialization models. First, supply-chain diversification and nearshoring of critical biologic production should be pursued to reduce exposure to tariff-driven cost volatility and logistical disruption, while investment in cold-chain and lot-release redundancy will protect continuity of care. Second, development programs should emphasize patient-centric endpoints, real-world evidence generation, and head-to-head or pragmatic comparative studies that speak directly to payer value frameworks. These evidence investments will facilitate negotiation and support favorable formulary placement.Third, companies should accelerate work on administration modalities that lower treatment burden, including subcutaneous and oral platforms, and concurrently design comprehensive patient support and adherence programs to maximize real-world effectiveness. Fourth, engage proactively with payers, clinicians, and patient groups to co-design access solutions that balance affordability with innovation incentives, including outcomes-based agreements where appropriate. Finally, cultivate strategic alliances across manufacturing, distribution, and digital health partners to enhance speed-to-market, optimize cost structures, and enable differentiated service offerings that improve patient outcomes and strengthen competitive positioning.
A rigorous mixed-methods research methodology integrating expert interviews, clinical literature review, and real-world data synthesis to ensure robust and actionable HAE insights
This analysis synthesizes primary qualitative inputs and secondary evidence through a structured methodology designed to triangulate clinical, commercial, and policy signals. Primary research included in-depth interviews with clinical experts, specialty pharmacists, payer representatives, and patient advocacy stakeholders to capture real-world treatment patterns, unmet needs, and access barriers. Secondary research involved systematic review of peer-reviewed literature, regulatory guidance documents, and product-level clinical data to validate mechanism-specific efficacy and safety narratives. Where possible, real-world datasets and registry information were consulted to contextualize treatment pathways and utilization patterns.The analytic approach applied a layered framework that mapped segmentation dimensions against clinical outcomes, route-of-administration implications, distribution models, and regional access variables. Evidence synthesis prioritized reproducibility and transparency, with assumptions and data provenance documented for stakeholder review. Limitations include potential variability in regional reporting and evolving policy landscapes that can shift access dynamics; accordingly, the methodology incorporates an update cadence for periodic refreshes and recommends targeted primary research in geographies or subsegments where data gaps remain significant.
A concise conclusion synthesizing critical opportunities and risks while urging coordinated action across clinical, commercial, and policy stakeholders to advance patient outcomes
In conclusion, the therapeutic and commercial landscape for hereditary angioedema is at an inflection point driven by pharmacologic innovation, evolving delivery models, and shifting payer expectations. Stakeholders that align product development with patient-centered convenience, invest in robust evidence programs that demonstrate total cost-of-care benefits, and proactively manage supply-chain and policy risks will capture disproportionate advantage. The interplay of route of administration, mechanism-specific differentiation, distribution pathways, and regional regulatory nuance creates multiple levers for value creation that must be orchestrated cohesively.Looking ahead, the imperative for manufacturers is to translate clinical differentiation into accessible, affordable therapies through strategic partnerships, targeted evidence generation, and adaptive commercial models. Payers and providers should work collaboratively with manufacturers to design access frameworks that preserve innovation incentives while ensuring sustainable care delivery. Ultimately, the most successful strategies will be those that center on patient outcomes, operational resilience, and transparent engagement across the health system ecosystem.
Table of Contents
18. ResearchStatistics
19. ResearchContacts
20. ResearchArticles
21. Appendix
Companies Mentioned
- ADARx Pharmaceuticals, Inc.
- Arrowhead Pharmaceuticals, Inc.
- Astria Therapeutics, Inc.
- Attune Pharmaceuticals, Inc.
- BioCryst Pharmaceuticals, Inc.
- BioMarin Pharmaceutical Inc.
- CSL Behring LLC
- Grifols, S.A.
- Intellia Therapeutics, Inc.
- Ionis Pharmaceuticals, Inc.
- KalVista Pharmaceuticals, Inc.
- Pharming Group N.V.
- Pharvaris N.V.
- Regeneron Pharmaceuticals, Inc.
- Sanofi S.A.
- Swedish Orphan Biovitrum AB
- Takeda Pharmaceutical Company Limited
- Teva Pharmaceuticals Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 186 |
| Published | January 2026 |
| Forecast Period | 2026 - 2032 |
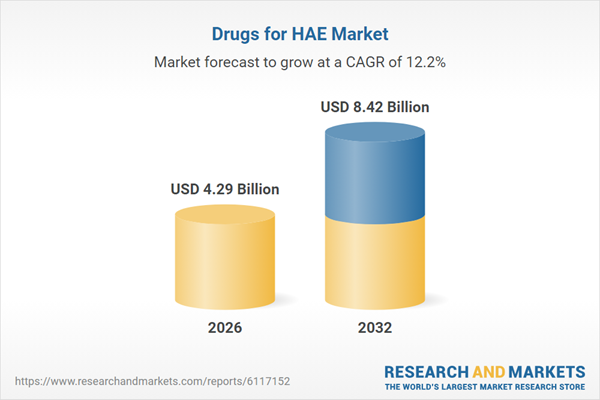
| Estimated Market Value ( USD | $ 4.29 Billion |
| Forecasted Market Value ( USD | $ 8.42 Billion |
| Compound Annual Growth Rate | 12.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 18 |